| Cas No.: | 24386-93-4 |
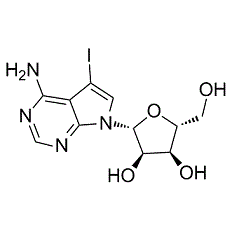
| Chemical Name: | 5-Iodo-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidin-4- amine |
| Synonyms: | 5-Iodo-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidin-4- amine |
| SMILES: | NC1=C2C(N([C@H]3[C@H](O)[C@H](O)[C@@H](CO)O3)C=C2I)=NC=N1 |
| Formula: | C11H13In4O4 |
| M.Wt: | 392.2 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | 5-Iodotubercidin (Itu) has been shown to inhibit mitogen-activated protein kinase (ERK2) (Ki = 525 nM), adenosine kinase (ADK) (Ki = 30 nM), casein kinases 1 & 2 (CSNK1A1 & CSNK2A1), protein kinase A (PKA) and insulin receptor kinase (IC50 ranging from 0. |
| In Vivo: | 5-Iodotubercidin (1 mL/kg, i.p.) is in agreement with activity observed against bicuculline-induced seizures following local administration of the AKI into the prepiriform cortex[2]. |
| In Vitro: | 5-Iodotubercidin (40 μM) enhances the rate of phosphorylase inactivation and shortens the lag before the activation of glycogen synthase. 5-Iodotubercidin (50 μM) antagonizes the effects of glucagon and vasopressin, but does not affect the basal concentration of free calcium in single hepatocytes[1]. 5-Iodotubercidin (20 μM) causes an important decrease in ATP concentration, and a concomitant smaller increase in AMP concentration. 5-Iodotubercidin decreases the activity of ACC and the rates of synthesis of fatty acids and cholesterol. In line with the iodotubercidin-mediated inhibition of ACC, 5-iodotubercidin induces a marked decrease in the intracellular concentration of malonyl-CoA[3]. 5-Iodotubercidin causes a strong decrease in the immunofluorescence levels of P-T3-H3, and depletion of P-T3-H3 is complete at 10 µM 5-5-iodotubercidin[4]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.