| Cas No.: | 162829-90-5 |
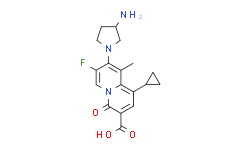
| Chemical Name: | 4H-Quinolizine-3-carboxylicacid, 8-[(3S)-3-amino-1-pyrrolidinyl]-1-cyclopropyl-7-fluoro-9-methyl-4-oxo- |
| Synonyms: | 4H-Quinolizine-3-carboxylicacid, 8-[(3S)-3-amino-1-pyrrolidinyl]-1-cyclopropyl-7-fluoro-9-methyl-4-oxo- |
| SMILES: | NC1CCN(C2=C(C)C3N(C(=O)C(C(O)=O)=CC=3C3CC3)C=C2F)C1 |
| Formula: | C18H20N3O3F |
| M.Wt: | 345.3681 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | ABT-719 is an S isomer and a representative 2-pyridone. ABT-719 administered orally or subcutaneously was 4- to 10-fold more effective than ciprofloxacin against Staphylococcus aureus, Streptococcus pneumoniae, and Streptococcus pyogenes infections in normal mice. ABT-719 was equivalent in efficacy to ciprofloxacin for treatment of gram-negative bacterial infections caused by Pseudomonas aeruginosa or Escherichia coli. The racemate and R forms of ABT-719 produced similar results against gram-positive and gram-negative bacterial infections. The 50% effective doses of ABT-719 were at least threefold lower than those of ciprofloxacin for therapy of intracellular infections caused by Salmonella typhimurium or Listeria monocytogenes. In immunosuppressed mice, ABT-719 was more effective than ciprofloxacin against quinolone-sensitive S. aureus, Enterococcus faecalis, and Enterococcus faecium. The pharmacokinetic properties of ABT-719 were consistent with its relative efficacy. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.