| Cas No.: | 248919-64-4 |
| Chemical Name: | Imidazo[1,2-a]pyridine-6-carboxamide,8-[[(2,6-dimethylphenyl)methyl]amino]-N-(2-hydroxyethyl)-2,3-dimethyl- |
| Synonyms: | Imidazo[1,2-a]pyridine-6-carboxamide,8-[[(2,6-dimethylphenyl)methyl]amino]-N-(2-hydroxyethyl)-2,3-dimethyl-;2,3-dimethyl-8-(2,6-dimethylbenzylamino)-N-hydroxyethyl-imidazo[1,2-a]pyridine-6-carboxamide;8-[(2,6-dimethylphenyl)methylamino]-N-(2-hydroxyethyl)-2,3-dimethylimidazo[1,2-a]pyridine-6-carboxamide;2,3-Dimethyl-8-[(2,6-dimethylbenzyl)amino]-6-[N-(2-hydroxyethyl)aminocarbonyl]imidazo[1,2-a]pyridine;AZD 0865;AZD-0865;Linaprazan |
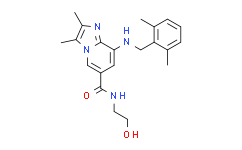
| SMILES: | O=C(C1=CN2C(C(NCC3=C(C)C=CC=C3C)=C1)=NC(C)=C2C)NCCO |
| Formula: | C21H26N4O2 |
| M.Wt: | 366.45674 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | AZD0865 inhibits gastric H+,K+-ATPase by K+-competitive binding. (IC50: 1.0 ± 0.2 μM)It is a acid-suppressing agents with rapid onset of action and potent acid inhibition. |
| In Vitro: | AZD0865 can inhibit the final step in acid secretion. AZD0865 reduced porcine renal Na+,K+-ATPase activity by 9 ± 2%, demonstrating a high selectivity for H+,K+-ATPase.In vivo: The reference for animal administration is 0.5-1.0 mg/kg. The greater degree of acid suppression with the 75-mg dose of AZD0865 would translate to a healing rate of 89% at 4 weeks. |
| References: | [1]. Gedda K et al. Mechanism of action of AZD0865, a K+-competitive inhibitor of gastric H+,K+-ATPase.Biochem Pharmacol. 2007 Jan 15;73(2):198-205. [2]. Kahrilas PJ et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007 Dec;5(12):1385-91. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.