| Cas No.: | 224047-41-0 |
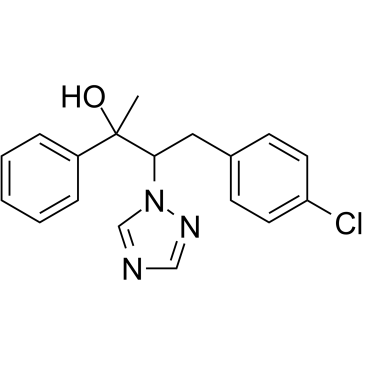
| Chemical Name: | 4-(4-chlorophenyl)-2-phenyl-3-(1H-1,2,4-triazol-1-yl)butan-2-ol |
| Synonyms: | Brassinazole , |
| SMILES: | OC(C1=CC=CC=C1)(C)C(CC2=CC=C(Cl)C=C2)N3N=CN=C3 |
| Formula: | C18H18ClN3O |
| M.Wt: | 327.81 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | [1]. Y K Min, et al. New Lead Compounds for Brassinosteroid Biosynthesis Inhibitors. Bioorg Med Chem Lett. 1999 Feb 8;9(3):425-30. [2]. Eriko Sasaki, et al. Uniconazole, a Cytochrome P450 Inhibitor, Inhibits Trans-Zeatin Biosynthesis in Arabidopsis. Phytochemistry. 2013 Mar;87:30-8. |
| Description: | (Rac)-Brassinazole, triazole-type compound, is a brassinosteroid (BR) biosynthesis inhibitor. (Rac)-Brassinazole increases inhibition of CYP90B in BR biosynthesis[1][2] |
| In Vitro: | (Rac)-Brassinazole (compound 5) does not retard rice stem elongation, and retardation is recovered by the addition of gibberellin (GA3), suggesting that such retardation is due to the inhibition of gibberellin biosynthesis[1]. (Rac)-Brassinazol (compound 31) results in a reduction in the effect on AtKO in gibberellin (GA) biosynthesis[2]. |
| References: | [1]. Y K Min, et al. New Lead Compounds for Brassinosteroid Biosynthesis Inhibitors. Bioorg Med Chem Lett. 1999 Feb 8;9(3):425-30. [2]. Eriko Sasaki, et al. Uniconazole, a Cytochrome P450 Inhibitor, Inhibits Trans-Zeatin Biosynthesis in Arabidopsis. Phytochemistry. 2013 Mar;87:30-8. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.