| Cas No.: | 851107-28-3 |
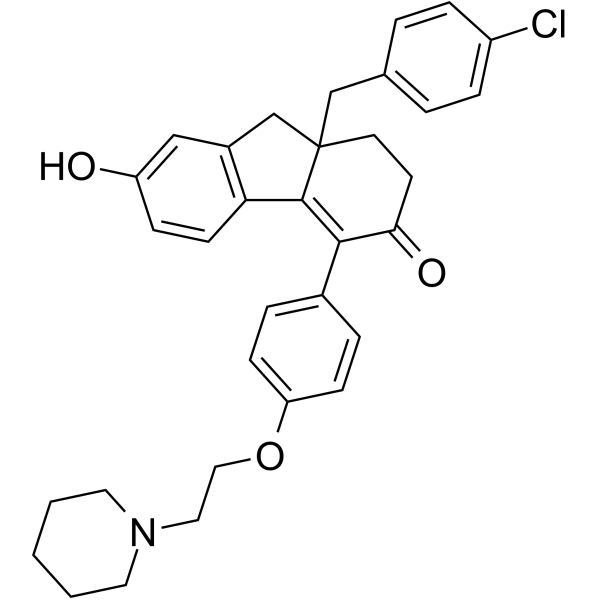
| Chemical Name: | 9a-[(4-Chlorophenyl)methyl]-7-hydroxy-4-[4-(2-piperidin-1-ylethoxy)phenyl]-2,9-dihydro-1H-fluoren-3-one |
| Synonyms: | 9a-(4-chlorobenzyl)-7-hydroxy-4-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-1,2,9,9a-tetrahydro-3H-fluoren-3-one;CMP8;9a-(4-Chlorobenzyl)-7-hydroxy-4-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-9,9a-dihydro-1H-fluoren-3(2H)-one;CMP-8;BCP20255;BDBM50194075;9a-(4-chlorobenzyl)-7-hydroxy-4-[4-(2-piperidin-1-ylethoxy)phenyl]-1,2,9,9a-tetrahydro-3H-fluoren-3-one;9a-[(4-Chlorophenyl)methyl]-7-hydroxy-4-[4-(2-piperidin-1-ylethoxy)phenyl]-2,9-dihydro-1H-fluoren-3-one |
| SMILES: | ClC1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])C12C([H])([H])C([H])([H])C(C(C3C([H])=C([H])C(=C([H])C=3[H])OC([H])([H])C([H])([H])N3C([H])([H])C([H])([H])C([H])([H])C([H])([H])C3([H])[H])=C1C1C([H])=C([H])C(=C([H])C=1C2([H])[H])O[H])=O |
| Formula: | C33H34ClNO3 |
| M.Wt: | 528.0810 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | CMP8, a selective ligand for estrogen receptor, binds to the mutant estrogen receptor ligand binding domain (ERLBD). CMP8 exhibits IC50 values of 29 nM , 41 nM, 1100 nM and 2200 nM for MGERα, MGRERα, hERα and hERβ, respectively[1][2]. |
| In Vitro: | CMP8 (Compound 20h) is dosed in male Balb-c mice (4 mg/kg, ip). The plasma concentration after 4 h was 1.5-fold its EC50 in mammalian cells with a Cmax of 0.5 µM after 30 min[1][2]. |
| References: | [1]. Miyazaki Y, et al. Destabilizing domains derived from the human estrogen receptor. J Am Chem Soc. 2012 Mar 7;134(9):3942-5. [2]. Kinzel O, et al. A structure-guided approach to an orthogonal estrogen-receptor-based gene switch activated by ligands suitable for in vivo studies. J Med Chem. 2006 Sep 7;49(18):5404-7. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.