| Cas No.: | 928672-86-0 |
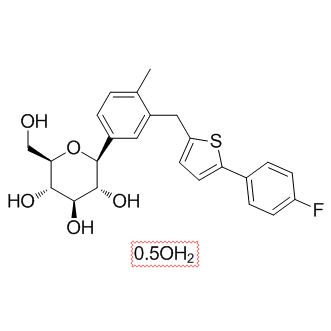
| Chemical Name: | (3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol hemihydrate |
| Synonyms: | JNJ 28431754; JNJ-28431754; JNJ28431754; TA 7284; TA-7284; TA7284; JNJ-24831754-ZAE; JNJ-28431754-AAA; Canagliflozin hemihydrate; brand name: Invokana. Sulisent. |
| SMILES: | CC1=CC=C([C@H]2[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O2)C=C1CC3=CC=C(C4=CC=C(F)C=C4)S3.[0.5OH2] |
| Formula: | C48H52F2O11S2 |
| M.Wt: | 907.0498 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Canagliflozin 0.5 H2O(JNJ 28431754; TA 7284) is a highly potent and selective SGLT2 inhibitor for hSGLT2 with IC50 of 2.2 nM, exhibits 413-fold selectivity over hSGLT1. IC50 value: 2.2 nM Target: SGLT2 Canagliflozin(JNJ 24831754ZAE; JNJ 28431754; JNJ 28431754AAA; TA 7284) is an experimental drug being developed by Johnson & Johnson for the treatment of type 2 diabetes.Canagliflozin(JNJ 24831754ZAE; JNJ 28431754; JNJ 28431754AAA; TA 7284) is an inhibitor of subtype 2 sodium-glucose transport protein (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine.For the detailed information of Canagliflozin hemihydrate, the solubility of Canagliflozin hemihydrate in water, the solubility of Canagliflozin hemihydrate in DMSO, the solubility of Canagliflozin hemihydrate in PBS buffer, the animal experiment (test) of Canagliflozin hemihydrate, the cell expriment (test) of Canagliflozin hemihydrate, the in vivo, in vitro and clinical trial test of Canagliflozin hemihydrate, the EC50, IC50,and affinity,of Canagliflozin hemihydrate, Please contact DC Chemicals. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.