| Cas No.: | 1092970-12-1 |
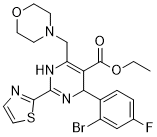
| Chemical Name: | Morphothiadin |
| Synonyms: | Morphothiadin;ethyl 4-(2-bromo-4-fluorophenyl)-6-(morpholin-4-ylmethyl)-2-(1,3-thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate;ethyl 4-[2-bromo-4-fluorophenyl]-6-[morpholino-methyl]-2-[2-thiazolyl]-1,4-dihydro-pyrimidine-5-carboxylate;Morphothiadine;Morphothiadine [WHO-DD];GLS-4;GLS4; GLS-4; GLS 4;BCP29837;BDBM50247812;5-Pyrimidinecarboxylic acid, 4-(2-bromo-4-fluorop;GLS4 |
| SMILES: | BrC1C=C(C=CC=1C1C(C(=O)OCC)=C(CN2CCOCC2)NC(C2=NC=CS2)=N1)F |
| Formula: | C21H22BrFN4O3S |
| M.Wt: | 509.39178609848 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | GLS4 (Morphothiadin) is a potent inhibitor of HBV capsid assembly, inhibits HBV replication (EC50=62.24 nM) and reduces HBV-DNA levels in HepG.2.2.15 cells (IC50=14 nM), shows efficacy against ADV-resistant HBV mutations; strongly inhibits core gene expression (at 100 to 200 nM), suppresses virus accumulation in the supernantant of HepAD38 cells; inhibits HBV replicative forms in the live, shows strong and sustained suppression of virus DNA in treated mice.HBV InfectionPhase 3 Clinical |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.