| Cas No.: | 1535212-07-7 |
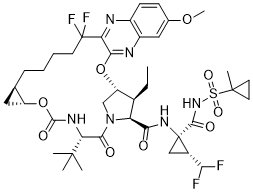
| Chemical Name: | (33R,34S,35S,91R,92R,5S)-5-(tert-butyl)-N-((1R,2R)-2-(difluoromethyl)-1-(((1-methylcyclopropyl)sulfonyl)carbamoyl)cyclopropyl)-34-ethyl-14,14-difluoro-17-methoxy-4,7-dioxo-2,8-dioxa-6-aza-1(2,3)-quinoxalina-3(3,1)-pyrrolidina-9(1,2)-cyclopropanacyclotetra |
| Synonyms: | GS-9857;GS9857 |
| SMILES: | CC[C@@H]1[C@@H]2CN([C@@H]1C(=O)N[C@@]3(C[C@H]3C(F)F)C(=O)NS(=O)(=O)C4(CC4)C)C(=O)[C@@H](NC(=O)O[C@@H]5C[C@H]5CCCCC(C6=NC7=C(C=C(C=C7)OC)N=C6O2)(F)F)C(C)(C)C |
| Formula: | C40H52F4N6O9S |
| M.Wt: | 868.94 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Voxilaprevir (GS-9857) is a fluorinated macrocyclic hepatitis C virus (HCV) nonstructural protein (NS) 3/4A protease inhibitor with potent in vitro antiviral activity against genotypes 1-6 HCV and broad coverage of NS3/4A protease polymorphisms. GS-9857 improves coverage against commonly encountered NS3 resistance-associated variants (RAVs)[1][2]. |
| Target: | Hepatitis C virus (HCV) nonstructural protein (NS) 3/4A protease[1] |
| References: | [1]. Rodriguez-Torres M, et al. GS-9857 in patients with chronic hepatitis C virus genotype 1-4 infection: a randomized, double-blind, dose-ranging phase 1 study. J Viral Hepat. 2016 Aug;23(8):614-22. [2]. Lawitz E, et al. Efficacy of Sofosbuvir, Velpatasvir, and GS-9857 in Patients With Genotype 1 Hepatitis C VirusInfection in an Open-Label, Phase 2 Trial. Gastroenterology. 2016 Nov;151(5):893-901.e1. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.