| Cas No.: | 163706-36-3 |
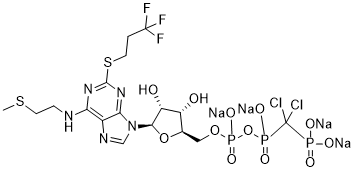
| Chemical Name: | (dichloro((((((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-((2-(methylthio)ethyl)amino)-2-((3,3,3-trifluoropropyl)thio)-9H-purin-9-yl)tetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)oxy)(hydroxy)phosphoryl)methyl)phosphonic acid, tetrasodium salt |
| Synonyms: | AR-C69931MX; AR C69931MX; ARC69931MX; AR-C69931; AR C69931; ARC69931; Cangrelor; Cangrelor tetrasodium salt |
| SMILES: | O=P(C(Cl)(P(O[Na])(O[Na])=O)Cl)(OP(OC[C@H]1O[C@@H](N2C=NC3=C(NCCSC)N=C(SCCC(F)(F)F)N=C23)[C@H](O)[C@@H]1O)(O[Na])=O)O[Na] |
| Formula: | C17H21Cl2F3N5Na4O12P3S2 |
| M.Wt: | 864.29 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Cangrelor tetrasodium, an adenosine triphosphate analogue, is a reversible and selective platelet P2Y12 antagonist, with prompt and potent antiplatelet effects. Cangrelor tetrasodium directly blocks adenosine diphosphate (ADP)-induced activation and aggregation of platelets. Cangrelor tetrasodium is also a nonspecific GPR17 antagonist[1][2]. |
| In Vivo: | Cangrelor tetrasodium (10 mg/kg) not only significantly decreases BLM-induced release of inflammatory cytokines (PF4, CD40 L and MPO), but also decreases the increment of platelets, neutrophils and platelet-neutrophil aggregates in the fibrotic lung and in the peripheral blood of BLM-treated mice[2]. |
| In Vitro: | Cangrelor tetrasodium is the only potent intravenous direct and specific adenosine diphosphate (ADP) P2Y12 receptor antagonist[1]. Cangrelor tetrasodium has pKb of 8.6-9.2 for hP2Y12 receptor[3]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.