| Cas No.: | 168482-23-3 |
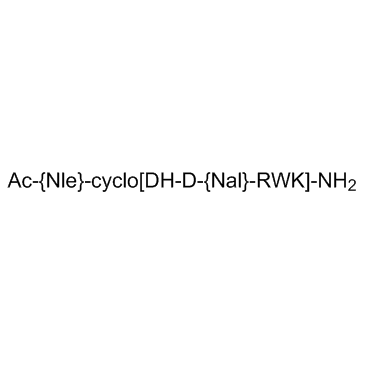
| Chemical Name: | Nle(Ac)-cyclo(Asp-His-D-2-Nal-Arg-Trp-Lys)-NH2 |
| Synonyms: | SHU9119 |
| SMILES: | N/C(=N/CCC[C@@H](C(N[C@@H](CC1CNC2=CC=CC=C12)C(NC1=CCCCNC(=O)C[C@H](NC([C@@H](NC(=O)C)CCCC)=O)C(=O)N[C@@H](CC2C=NC=N2)C(=O)NC1=O)=O)=O)NC([C@@H](CC1C=CC2=CC=CC=C2C=1)N)=O)/N |c:24,&1:6,9,31,34,47,63| |
| Formula: | C54H71N15O9 |
| M.Wt: | 1074.258 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | [1]. Grieco P, et al. Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides. 2007 Jun;28(6):1191-6. [2]. Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007 Nov;117(11):3475-88. [3]. Kooijman S, et al. Inhibition of the central melanocortin system decreases brown adipose tissue activity. J Lipid Res. 2014 Oct;55(10):2022-32. |
| Description: | SHU 9119 is a potent human melanocortin 3 and 4 receptors (MC3/4R) antagonist and a partial MC5R agonist; with IC50 values of 0.23, 0.06, and 0.09 nM for human MC3R, MC4R and MC5R, respectively. |
| Target: | IC50: 0.23 nM (MC3R); 0.23 nM (MC4R); 0.23 nM (MC5R)[1] |
| In Vivo: | Blockade of CNS-Mcr via chronic intracerebroventricular infusion of SHU9119 (24 nmol/d for 7 days) increases food intake in ad libitum-fed rats compared with control. Weight gain of SHU9119 treated rats is significantly higher than control. SHU9119 treatment potently increases metabolic efficiency. SHU9119 markedly increases mRNA levels of genes promoting lipogenesis and TAG storage in adipocytes, including stearoyl-CoA desaturase-1, lipoprotein lipase, acetyl-CoA carboxylase α, and fatty acid synthase[2]. SHU9119 increases food intake (+30%) and body fat (+50%) and decreases EE by reduction in fat oxidation (−42%). In addition, SHU9119 impairs the uptake of VLDL-TG by BAT. In line with this, SHU9119 decreases uncoupling protein-1 levels in BAT (−60%) and induces large intracellular lipid droplets, indicative of severely disturbed BAT activity[3]. |
| Animal Administration: | Rats: SHU9119 is prepared in saline. SHU9119 is infused i.c.v. at a concentration of 24 nM and MTII at a concentration of 1 nM per day for 2 or 7 days. To differentiate between SHU9119 effects per se from those related to increased food intake, we prevent overeating in a group of SHU9119-treated rats by pair-feeding, so that they are given the same amount of food as consumed by a control group (SHU9119-pf). The pair-feeding regimen consists in giving one-third of the daily food amount in the morning and the remaining two-thirds just before onset of darkness. Thus, most of the experiments include 5 groups of animals: an ad libitum–fed control group infused with i.c.v. saline; 2 i.c.v. SHU9119-infused groups: SHU9119–ad lib and SHU9119-pf; an MTII-infused group; and a saline-pf group[2]. Mice: SHU9119 is prepared in artificial cerebrospinal fluid. After 4 weeks of run-in diet, mice are randomized into groups that received icv administration of artificial cerebrospinal fluid (vehicle) or SHU9119 (5 nmol/day) in vehicle during 14-17 days. The effect of SHU9119 on BAT activity independent of food intake is also investigated by using an additional SHU9119-treated group that is pair-fed (SHU9119-pf) to the vehicle-treated group. To achieve pair-feeding, food intake of the ad libitum-fed mice is monitored daily, and pair-fed mice receive surgery 1 day behind the control mice. The pair-feeding regimen consisted of giving the mice the average daily consumed food amount by the control mice, just before onset of darkness[3]. |
| References: | [1]. Grieco P, et al. Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides. 2007 Jun;28(6):1191-6. [2]. Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007 Nov;117(11):3475-88. [3]. Kooijman S, et al. Inhibition of the central melanocortin system decreases brown adipose tissue activity. J Lipid Res. 2014 Oct;55(10):2022-32. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.