| Cas No.: | 215030-90-3 |
| Chemical Name: | BMS 493 |
| Synonyms: | BMS 493;4-[(1E)-2-[5,6-Dihydro-5,5-diMethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoicacid;(E)-4-[2-[5,6-Dihydro-5,5-dimethyl-8-(2-phenylethynyl)naphthalen-2-yl]ethen-1-yl]benzoic acid;4-[(1E)-2-[5,6-Dihydro-5,5-dimethyl-8-(phenylethynyl)-2-naphthalenyl]ethenyl]-benzoic acid;BMS204, 493 |
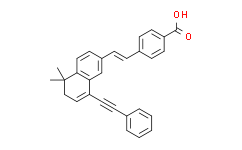
| SMILES: | OC(C1=CC=C(/C=C/C2C=CC3C(CC=C(C#CC4=CC=CC=C4)C=3C=2)(C)C)C=C1)=O |
| Formula: | C29H24O2 |
| M.Wt: | 404.499668121338 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | BMS-204493(BMS493) is an inverse pan-retinoic acid receptor (RAR) agonist. BMS493 increases nuclear corepressor interaction with RARs. BMS493 also could prevent retinoic acid-induced differentiation[1][2]. |
| Target: | RAR |
| In Vivo: | Intrapancreatic transplantation of cell progeny after expansion of ALDHhi cells with or without BMS493 shows no reduction of hyperglycemia in Streptozotocin-treated NOD/SCID mice. Thus, Umbilical cord blood (UCB)-derived ALDHhi cells effectively lost islet regenerative capacity during ex vivo expansion[1]. |
| In Vitro: | BMS493 (100 nM; 6 days; ALDHhi UCB cells) treatment shows a twofold increase in the number of ALDHhi cells available for transplantation compared with untreated controls. Newly expanded ALDHhi cells shows increased numbers of CD34 and CD133-positive cells, as well as a reduction in CD38 expression, a marker of hematopoietic cell differentiation[1]. Cell Viability Assay[1] Cell Line: ALDHhi UCB cells Concentration: 100 nM Incubation Time: 6 days Result: Showed a twofold increase in the number of ALDHhi cells available for transplantation compared with untreated controls. |
| References: | [1]. Elgamal RM, et al. BMS 493 Modulates Retinoic Acid-Induced Differentiation During Expansion of Human Hematopoietic Progenitor Cells for Islet Regeneration. Stem Cells Dev. 2018 Aug 1;27(15):1062-1075. [2]. Yu Z, et al. Apoptosis induced by atRA in MEPM cells is mediated through activation of caspase and RAR. Toxicol Sci. 2006 Feb;89(2):504-9. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.