| Cas No.: | 1397922-61-0 |
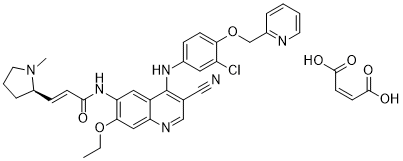
| Chemical Name: | (R,E)-N-(4-((3-chloro-4-(pyridin-2-ylmethoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-3-(1-methylpyrrolidin-2-yl)acrylamide dimaleate |
| Synonyms: | SHR-1258 maleate;SHR1258 maleate |
| SMILES: | CCOC1=C(C=C2C(=C(C=NC2=C1)C#N)NC3=CC(=C(C=C3)OCC4=CC=CC=N4)Cl)NC(=O)/C=C/[C@@H]5N(CCC5)C.C(=C\C(=O)O)\C(=O)O.C(=C\C(=O)O)\C(=O)O |
| Formula: | C36H35ClN6O7 |
| M.Wt: | 699.161 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Pyrotinib dimaleate (SHR-1258 dimaleate) is a potent and selective EGFR/HER2 dual inhibitor with IC50 s of 13 and 38 nM, respectively. |
| In Vivo: | Pyrotinib dimaleate has acceptable bioavailability of 20.6%, 43.5% and 13.5% in nude mice, rats and dogs, respectively. Pyrotinib dimaleate has favorable drug-like physicochemical properties and shows relatively higher oral exposure in human subjects (oral; t1/2=15 h) with a much longer half life than that of preclinical animal species such as mouse (i.v.; t1/2=1.56 h; i.g.; t1/2=2.52 h) and rat (i.v.; t1/2=4.42 h; i.g.; t1/2=3.38 h)[1]. |
| In Vitro: | Pyrotinib dimaleate has high potency in HER2-dependent cell lines (BT474, SK-OV-3), while showing much weaker inhibition in the HER2 negative cell line (MDA-MB-231). Pyrotinib dimaleate inhibits BT474 and SK-OV-3 cells with IC50s of 5.1 and 43 nM, respectively. Pyrotinib dimaleate displays high selectivity as HKI-272 when tested in a panel of different kinases such as KDR, c-Kit, PDGFRβ, c-Src and C-Met (c-Src with an IC50 of 790 nM, and others >3000 nM)[1]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.