| Cas No.: | 130929-57-6 |
| Chemical Name: | Entacapone |
| Synonyms: | Entacapone;2-cyano-3-(5-dihydroxyamino-3,4-dioxo-1-cyclohexa-1,5-dienyl)-n,n-diethyl-prop-2-enamide;2-Cyano-N,N-diethyl-3-(3,4-dihydroxy-5-nitrophenyl)propenamide;Comtan;Comtes;Comtess;OR-611;ENTACAPONE F·S;Entacapone (150 mg);Entacapone (isomer E);(E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylacrylamide;(E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-pmpenamide |
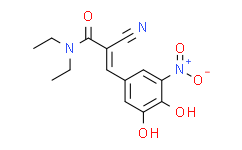
| SMILES: | O=C(N(CC)CC)/C(C#N)=C/C1=CC([N+]([O-])=O)=C(O)C(O)=C1 |
| Formula: | C14H15N3O5 |
| M.Wt: | 305.29 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Entacapone is a specific, potent, peripherally acting catechol-O-methyltransferase (COMT) inhibitor with IC50 of 151 nM for PD treatment.IC50 Value: 151 nMTarget: COMTin vitro: Entacapone inhibits catechol-O-methyltransferase(COMT) with similar IC50 in different tissues including live, duodenum, kidney and lung, but entacapone is more active than tolcapone in those tissues. Entacapone (< 100 μM) is a potent inhibitor of α-syn and β-amyloid (Aβ) oligomerization and fibrillogenesis, and also protects against extracellular toxicity induced by the aggregation of both proteins in PC12 cells.in vivo: Levodopa/carbidopa/entacapone has been shown to improve the pharmacokinetic profile of levodopa and provide superior symptomatic control compared with conventional levodopa/dopa decarboxylase inhibitor therapy. We report four case histories describing clinical experience of using levodopa/carbidopa/entacapone 200/50/200 mg, one of the latest doses of this formulation, in a range of patients with Parkinson's disease. These cases illustrate that levodopa/carbidopa/entacapone 200/50/200 mg provides improvements in symptomatic control.Clinical trial: The combination product carbidopa/levodopa/entacapone (CLE) was approved in 2003 for the treatment of PD patients. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.