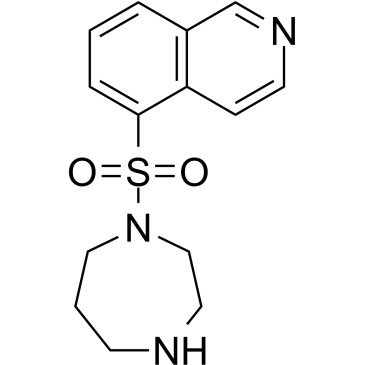
| Description: |
Fasudil is a potent inhibitor of ROCK1, PKA, PKC, and MLCK with Ki of 0.33 μM, 1.0 μM, 9.3 μM and 55 μM, respectively. |
| Target: |
p160ROCK:0.33 μM (Ki)
PKA:1 μM (Ki)
PKC:9.3 μM (Ki)
MLCK:55 μM (Ki) |
| In Vivo: |
Fasudil (30 μg) increases CBF by 50% via intra-coronary injection to dogs. Fasudil (0.01, 0.03, 0.1 and 0.3 mg/kg, bolus, i.v.) decreases MBP and increases HR, VBF, CBF, RBF, and FBF. Fasudil (1.0 ng/mL) increases cardiac output. Fasudil via i.v. produces a significant fall in MBP, left ventricular systolic pressure and total peripheral resistance with an increase in HR and cardiac output, but without obvious effect on right atrial pressure, dP/dt or left ventricular minute work in dogs[3]. Fasudil exhibits protectable effects on cardiovascular disease and reduces the activation of JNK and attenuates mitochondrial-nuclear translocation of AIF under ischemic injury[6]. Fasudil (100 mg/kg/day, p.o.) significantly reduces incidence and mean maximum clinical score of EAE in SJL/J mice immunized with PLP p139-151. Fasudil inhibits the proliferative response of splenocytes to the antigen in mice. Fasudil decreases inflammation, demyelination, axonal loss and APP positivein spinal cord of Fasudil-treated mice via p.o. administration[7]. |
| In Vitro: |
Fasudil has vasodilatory action and occupies the adenine pocket of the ATP-binding site of the enzyme[1]. Fasudil produces a competitive inhibition of the Ca2+-induced contraction of the depolarized rabbit aorta. Fasudil inhibits contractile responses to KCl, phenylephnne (PHE) and prostaglandin (PG) F2a[2]. Fasudil also exhibits vasodilator actions by inhibition of 5-hydroxytryptamine, noradrenaline, histamine, angiotensin, and dopamine induced spiral strips contraction[3]. In addition, Fasudil induces disorganization of actin stress fiber and cell migration inhibition[4]. Fasudil inhibits hepatic stellate cells spreading, the formation of stress fibers, and expression of α-SMA with concomitant suppression of cell growth, but does not induce apoptosis. Fasudil also blocks the LPA-induced phosphorylation of ERK1/2, JNK and p38 MAPK[5]. |
| Kinase Assay: |
Cyclic AMP-dependent protein kinase activity is assayed in a reaction mixture containing, in a final volume of 0.2 mL, 50 mM Tris-HCl (pH 7.0), 10 mM magnesium acetate, 2 mM EGTA, 1 μM cyclic AMP or absence of cyclic AMP, 3.3 to 20 μM [r-32P] ATP (4×105 c.p.m.), 0.5 μg of the enzyme, 100 μg of histone H2B and compound. The mixture is incubated at 30°C for 5 min. The reaction is terminated by adding 1mL of ice-cold 20% trichloroacetic acid after adding 500 μg of bovine serum albumin as a carrier protein. The sample is centrifuged at 3000 r.p.m. for 15min, the pellet is resuspended in ice-cold 10% trichloro-acetic acid solution and the centrifugation-resuspension cycle is repeated three times. The final pellet is dissolved in 1 mL of 1 N NaOH and radioactivity is measured with a liquid scintillation counter[1]. |
| References: |
[1]. Ono-Saito N, et al. H-series protein kinase inhibitors and potential clinical applications. Pharmacol Ther. 1999 May-Jun;82(2-3):123-31.
[2]. Asano T, et al. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987 Jun;241(3):1033-40.
[3]. Asano T, et al. Vasodilator actions of HA1077 in vitro and in vivo putatively mediated by the inhibition of protein kinase. Br J Pharmacol. 1989 Dec;98(4):1091-100.
[4]. Negoro N, et al. The kinase inhibitor fasudil (HA-1077) reduces intimal hyperplasia through inhibiting migration and enhancing cell loss of vascular smooth muscle cells. Biochem Biophys Res Commun. 1999 Aug 19;262(1):211-5.
[5]. Fukushima M, et al. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005 Aug;25(4):829-38.
[6]. Zhang J, et al. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin Chim Acta. 2009 Mar;401(1-2):76-80.
[7]. Sun X, et al. The selective Rho-kinase inhibitor Fasudil is protective and therapeutic in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006 Nov;180(1-2):126-34.
[8]. Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997 Oct 30;389(6654):990-4. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.