| Cas No.: | 1229208-44-9 |
| Chemical Name: | Entospletinib |
| Synonyms: | Entospletinib (GS-9973);GS-9973;6-(1H-indazol-6-yl)-N-(4-morpholin-4-ylphenyl)imidazo[1,2-a]pyrazin-8-amine;6-(1H-indazol-6-yl)-N-(4-morpholinophenyl)imidazo[1,2-a]pyrazin-8-amine;Entospletinib;Entospletinib (GS-9973);GS9973;Entospletinib [INN];S7523;UNII-6I3O3W6O3B;6-(1H-Indazol-6-yl)-N-[4-(4-morpholinyl)phenyl]imidazo[1,2-a]pyrazin-8-amine;GS 9973 |
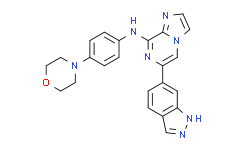
| SMILES: | C12=NC=CN1C=C(C3=CC4=C(C=C3)C=NN4)N=C2NC5=CC=C(N6CCOCC6)C=C5 |
| Formula: | C23H21N7O |
| M.Wt: | 411.46 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Entospletinib (GS-9973) is an orally bioavailable, selective?Syk inhibitor with an IC50 of 7.7 nM. |
| In Vivo: | Entospletinib (GS-9973) (1 mg/kg, p.o.) shows moderate to high bioavailability in rat and dog. In a rat collagen-induced arthritis model, Entospletinib (GS-9973) (1-10 mg/kg, p.o.) significantly inhibits ankle inflammation. Moreover, Entospletinib (GS-9973) also shows disease-modifying activity in multiple histological measurements, including inhibition of pannus formation, cartilage damage, bone resorption, and peritosteal bone formation with ED50 ranging from 1.2 to 3.9 mg/kg[1]. |
| In Vitro: | Entospletinib (GS-9973) shows good bidirectional permeability across Caco-2 cell monolayers in vitro. In cells, Entospletinib (GS-9973) also shows excellent selectivity for Syk, and potently inhibits BCR-mediated activation and proliferation of B-cells as well as immune-complex-stimulated cytokine production in monocytes[1]. The combination of idelalisib and Entospletinib (GS-9973) synergistically inhibits CLL cell viability and further disrupts chemokine signaling[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.