| Cas No.: | 1346574-57-9 |
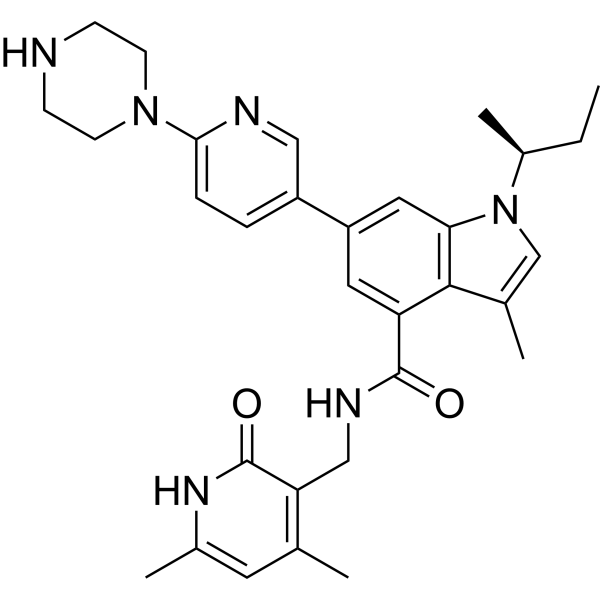
| Chemical Name: | (S)-1-(sec-butyl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-methyl-6-(6-(piperazin-1-yl)pyridin-3-yl)-1H-indole-4-carboxamide |
| Synonyms: | GSK-126,GSK 126,GSK126 |
| SMILES: | CC[C@H](C)N1C=C(C2=C(C=C(C=C21)C3=CN=C(C=C3)N4CCNCC4)C(=O)NCC5=C(C=C(NC5=O)C)C)C |
| Formula: | C31H38N6O2 |
| M.Wt: | 526.67 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | GSK126 is a potent, highly selective inhibitor of EZH2 methyltransferase with an IC50 of 9.9 nM. |
| In Vivo: | GSK126 is administered intraperitoneally at a dose volume of 0.2 mL per 20 g body weight in female beige SCID mice. GSK126 effectively inhibits the proliferation of EZH2 mutant DLBCL cell lines and markedly inhibits the growth of EZH2 mutant DLBCL xenografts in mice[1]. |
| In Vitro: | GSK126 potently inhibits both wild-type and mutant EZH2 methyltransferase activity with similar potencies (Ki=0.5-3 nM) independent of substrate used, and is competitive with S-adenosyl-methionine (SAM) and non-competitive with peptide substrates. GSK126 is highly selective against other methyltransferases and multiple other protein classes (EZH1, IC50=680 nM)[1]. Treatment of three SCLC cell lines with GSK126, induces growth inhibition. SCLC cell lines (Lu130, H209, and DMS53) are treated with 0.5, 2, and 8 μM GSK126, and growth curve is analyzed by WST-8 assay. Inhibition of cellular growth by GSK126 treatment is observed at 8 μM in all the three cell lines, while Lu130 and H209 are more sensitive to GSK126, even at lower doses[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.