| Cas No.: | 118525-40-9 |
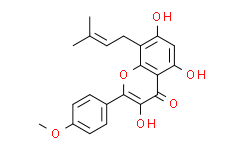
| Chemical Name: | Icaritin |
| Synonyms: | 4H-1-Benzopyran-4-one,3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-;3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromen-4-one;3,7-bis(2-hydroxyethyl)icaritin;Anhydroicaritin;Icaritin;3,7-dihydroxy-8-prenyl-4'-methoxychrysin;BIDD:ER0021;3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one;[ "" ];UFE666UELY;Cycloicaritin;Icartin;Anhydroicaritin;;Icaritin(Anhydroicaritin);MLS006010055;Icaritin, >=98% (HPLC);TUUXBSASAQJECY-UHFFFAOYSA-N;BDBM50272527;DB12672;AK16 |
| SMILES: | O1C(C2C([H])=C([H])C(=C([H])C=2[H])OC([H])([H])[H])=C(C(C2=C(C([H])=C(C(C([H])([H])/C(/[H])=C(\C([H])([H])[H])/C([H])([H])[H])=C12)O[H])O[H])=O)O[H] |
| Formula: | C21H20O6 |
| M.Wt: | 368.3799 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Icaritin(Anhydroicaritin) is a component of Epimedium flavonoid isolated from Herba Epimedii; enhances osteoblastic differentiation of mesenchymal stem cells (MSCs) while it inhibits adipogenic differentiation of MSCs by inhibiting PPAR-g pathway.IC50 value:Target: in vitro: Icaritin was unable to promote proliferation, migration and tube like structure formation by human umbilical vein endothelial cells (HUVECs) in vitro [1]. Icaritin potently inhibited proliferation of K562 cells (IC50 was 8 μM) and primary CML cells (IC50 was 13.4 μM for CML-CP and 18 μM for CML-BC), induced CML cells apoptosis and promoted the erythroid differentiation of K562 cells with time-dependent manner. Furthermore, Icaritin was able to suppress the growth of primary CD34+ leukemia cells (CML) and Imatinib-resistant cells, and to induce apoptosis [2]. icaritin strongly inhibited the growth of breast cancer MDA-MB-453 and MCF7 cells. At concentrations of 2-3 μM, icaritin induced cell cycle arrest at the G(2)/M phase accompanied by a down-regulation of the expression levels of the G(2)/M regulatory proteins such as cyclinB, cdc2 and cdc25C. Icaritin at concentrations of 4-5 μM, however, induced apoptotic cell death characterized by the accumulation of the annexin V- and propidium iodide-positive cells, cleavage of poly ADP-ribose polymerase (PARP) and down-regulation of the Bcl-2 expression [3].in vivo: In mouse leukemia model, Icaritin could prolong lifespan of NOD-SCID nude mice inoculated with K562 cells as effective as Imatinib without suppression of bone marrow. Icaritin could up-regulate phospho-JNK or phospho-C-Jun and down-regulate phospho-ERK, phospho-P-38, Jak-2, phospho-Stat3 and phospho-Akt expression with dose- or time-dependent manner [2]. |
| References: | [1]. Yao D, et al. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis--an in vitro efficacy study. PLoS One. 2012;7(8):e41264. [2]. Zhu Jf, et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3 /AKT signalings. PLoS One. 2011;6(8):e23720. [3]. Guo Y, et al. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol. 2011 May 11;658(2-3):114-22. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.