| Cas No.: | 170105-16-5 |
| Chemical Name: | Imidafenacin |
| Synonyms: | Imidafenacin;4-(2-Methyl-1H-imidazol-1-yl)-2,2-diphenylbutanamide;4-(2-methylimidazol-1-yl)-2,2-diphenylbutanamide;IMidafenacin DISCONTINUED-PATENTED PRODUCT;[14C]-Imidafenacin;4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide;KRP-197;ONO-8025;Staybla;Staybla (TN);Uritos;Uritos (TN);ONO 8025;2-Methyl-α,α-diphenyl-1H-iMidazole-1-butanaMide;1H-IMidazole-1-butanaMide,2-Methyl-a,a-diphenyl-;4-(2-Methyl-1-iMidazolyl)-2,2- diphenylbutanaMide;IMidafenacin DISCONTINUED-PATENTED PRODUCT |
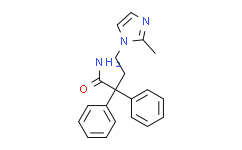
| SMILES: | NC(C(C1=CC=CC=C1)(C2=CC=CC=C2)CCN3C(C)=NC=C3)=O |
| Formula: | C20H21N3O |
| M.Wt: | 319.40024 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Imidafenacin(KRP-197; ONO-8025) is a potent and selective inhibitor of M3 receptors with Kb of 0.317 nM; less potent for M2 receptors(IC50=4.13 nM).IC50 value: 0.3 nM(M3) [1]in vitro: KRP-197 showed equipotent anti-M2 and anti-M3 activity and decreased subtype-selectivity [1]. in vivo: Intraduodenal administration of KRP-197 (0.04±0.30 mg/kg) inhibited bladder contraction dose-dependently, and the ED30 value was 0.11 mg/kg. The inhibitory action of KRP-197 on the bladder contraction was 19 times as potent as that of oxybutynin. KRP-197 showed preventive action againstthe decrease in bladder capacity induced by carbachol(ED50 0.074 mg/kg, intragastric administration), andthe potency of the inhibitory action was 15-fold greaterthan that of oxybutynin [1]. The learning-inhibitory doses of intravenous oxybutynin hydrochloride and tolterodine tartrate were 0.3 and 3 mg/kg in sham-operated rats and 0.1 and 1 mg/kg in nbM-lesioned rats, respectively. Thus, the learning impairments by those antimuscarinics were more sensitive in nbM-lesioned rats than in sham-operated rats. On the other hand, intravenous administration of imidafenacin had no influence on learning in either case of the rats. In normal rats, however, intracerebroventricular administration of imidafenacin impaired learning to the same degree as that of oxybutynin hydrochloride [2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.