| Cas No.: | 1052147-86-0 |
| Chemical Name: | GS-9674;GS9674;PX-104Cilofexor 1052147-86-0 |
| Synonyms: | 6-(4-Biphenyl-2-yl-piperazin-1-yl)-hexanoic acid 4-cyano-benzylamide;N-(4-Cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide;N-[(4-Cyanophenyl)methyl]-4-[1,1′-biphenyl]-2-yl-1-piperazinehexanamide;GS-9674;GS9674;PX-104Cilofexor 1052147-86-0;LP-211 |
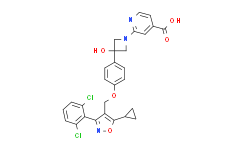
| SMILES: | ClC1C([H])=C([H])C([H])=C(C=1C1C(C([H])([H])OC2C([H])=C([H])C(=C([H])C=2[H])C2(C([H])([H])N(C3C([H])=C(C(=O)O[H])C([H])=C([H])N=3)C2([H])[H])O[H])=C(C2([H])C([H])([H])C2([H])[H])ON=1)Cl |
| Formula: | C28N3O5Cl2H23 |
| M.Wt: | 552.4053 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LP-211 is a selective and blood−brain barrier penetrant 5-HT7 receptor agonist, with a Ki of 0.58 nM, with high selectivity over 5-HT1A receptor (Ki, 188 nM) and D2 receptor (Ki, 142 nM). |
| In Vivo: | LP-211 (10 mg/kg, i.p.) rapidly reaches the systemic circulation in the mouse, with mean Cmax of 0.76 ± 0.32 μg/mL at 30 min[1]. LP-211 (0.003-0.3 mg/kg, i.p.) significantly increases the micturition volume in a dose-dependent manner, and causes significant increases in voiding efficiency in spinal cord-injured (SCI) rats, and such effects can be completely reversed by SB-269970[2]. LP-211 (0.25 and 0.50 mg/kg i.p.) improves consolidation of chamber-shape memory in rats, resulting in significant novelty-induced hyperactivity and recognition[3]. |
| In Vitro: | LP-211 is a selective 5-HT7 receptor agonist, with a Ki of 0.58 nM, 324- and 245-fold selectivity over 5-HT1A receptor (Ki, 188 nM) and D2 receptor (Ki, 142 nM). LP-211 shows agonist properties with an EC50 of 0.6 μM[1]. |
| Kinase Assay: | Binding of [3H]-LSD at rat cloned 5-HT7 receptor is performed in the assay. In 1 mL of incubation buffer (50 mM Tris, 10 mM MgCl2 and 0.5 mM EDTA, pH 7.4) are suspended 30 μg of membranes, 2.5 nM [3H]-LSD, LP-211 (6−9 concentrations). The samples are incubated for 60 min at 37°C. The incubation is stopped by rapid filtration on GF/A glass fiber filters (presoaked in 0.5% polyethylenimine for 30 min). The filters are washed with 3 × 53 mL of ice-cold buffer (50 mM Tris, pH 7.4). Nonspecific binding is determined in the presence of 10 μM 5-CT. Approximately 90% of specific binding is determined under these conditions[1]. |
| Animal Administration: | Rats[3] Thirty male adult Wistar rats (300-450 g) are assessed for novelty preference behavior after acute treatment (administered immediately after the training session and 24 h before the test session). After a 4 weeks' wash out, the rPDT is conducted to evaluate attraction from a greater/uncertain reward, with a sub-chronic treatment (five injections, immediately after sessions which follow the indifferent point). Food restriction, imposed by the experimenter through a limited quantity of food given at the end of each rPDT session, is applied to increase motivation to work for food delivery. All behavioral tests take place between 9:30 am and 4:00 pm. Rats are randomly assigned to treatment (LP-211 at 0.25 or 0.50 mg/kg i.p.) and control groups (injection volume 10 mL/kg; n = 10 per group). The brain penetrant 5-HT7R agonist LP-211 is dissolved in a vehicle solution of 1% dimethyl sulfoxide (DMSO) in saline (0.9% NaCl). Control group receives the vehicle strictly in the same conditions[3]. |
| References: | [1]. Leopoldo M, et al. Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J Med Chem. 2008 Sep 25;51(18):5813-22. [2]. Norouzi-Javidan A, et al. Effect of 5-HT7 receptor agonist, LP-211, on micturition following spinal cord injury in male rats. Am J Transl Res. 2016 Jun 15;8(6):2525-33. eCollection 2016. [3]. Beaudet G, et al. LP-211, a selective 5-HT7 receptor agonist, increases novelty-preference and promotes risk-prone behavior in rats. Synapse. 2017 Dec;71(12). |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.