| Cas No.: | 164083-84-5 |
| Chemical Name: | Phosphonic acid,P-[3-[[3-(2-amino-2-oxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-5-yl]oxy]propyl]- |
| Synonyms: | Phosphonic acid,P-[3-[[3-(2-amino-2-oxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-5-yl]oxy]propyl]-;LY 311727;Phosphonic acid,P-[3-[[3-(2-amino-2-oxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-5-yl]oxy]propy...;(3-{[3-(2-Amino-2-Oxoethyl)-1-Benzyl-2-Ethyl-1h-Indol-5-Yl]oxy}propyl)phosphonic Acid;[3-[[3-(2-AMino-2-oxoethyl)-2-ethyl-1-(phenylMethyl)-1H-indol-5-yl]oxy]propyl]-phosphonicacid;AC1L4F87;CHEMBL146186;CTK8E7560;LY-311727;SureCN7965980;(3-{[3-(2-amino-2-oxoethyl)-1-benzyl-2-ethyl-1H-indol-5-yl]oxy}propyl)phosphonic acid;[3-[[3-(2-Amino-2-oxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-5-yl]oxy]propyl]-phosphonicacid;(3-{[3-(2-amino-2-oxoethyl)-1-benzyl-2-ethyl-1H-indol-5-yl]oxy}propyl)phosphonic acid (ApexBio);Phosphonic acid, P-[3-[[3-(2-aMino-2-oxoethyl)-2-ethyl-1-(phenylMethyl)-1H-indol-5-yl]oxy]propyl]- |
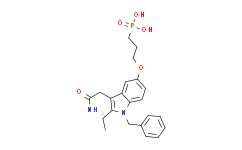
| SMILES: | NC(=O)CC1C2C(=CC=C(OCCCP(=O)(O)O)C=2)N(CC2C=CC=CC=2)C=1CC |
| Formula: | C22H27N2O5P |
| M.Wt: | 430.433946847916 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LY-311727 is a potent secretory non-pancreatic phospholipase A2 (sPLA2) inhibitor (IC50 <1 μM for group IIA sPLA2). sPLA2 is an important proinflammatory enzyme[1][2]. |
| Target: | sPLA2[1] |
| In Vivo: | LY-311727 (3-30 mg/kg; i.v.) dramatically suppresses the circulating enzyme activity in mice with Mt-sPLA2 transgenic the intravenous (i.v.) administration[2]. Animal Model: C57BL/6J mice, metallothionein promoter-human secretory PLA2 minigene (Mt-sPLA2) transgenic mice model[2] Dosage: 3 mg/kg, 10 mg/kg, 30 mg/kg Administration: Intravenous injection Result: Significantly and dose dependently suppressed the PLA2 activity in the serum. |
| In Vitro: | LY-311727 (0.1-10 μM) suppresses the contractile responses induced by human non-pancreatic secretory phospholipase A2 (hnps-PLA2), in a concentration related manner[1]. LY-311727 nearly abolishes the hnps-PLA2 responses at 1μM, while it failed to suppress porcine pancreatic PLA2 concentration response curves at the same concentration[1]. LY-311727 displays 1,500-fold selectivity when assayed against porcine pancreatic s-PLA2[1]. |
| References: | [1]. R W Schevitz, et al. Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat Struct Biol. 1995 Jun;2(6):458-65. [2]. N Fox, et al. Transgenic model for the discovery of novel human secretory non-pancreatic phospholipase A2 inhibitors. Eur J Pharmacol. 1996 Jul 18;308(2):195-203. [3]. M Murakami, et al. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998 Jun 5;273(23):14411-23. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.