| Cas No.: | 124489-20-9 |
| Chemical Name: | 2-Hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine |
| Synonyms: | N2-OH-PhIP; N2 OH PhIP; N2OHPhIP; N-Hydroxy PhIP; N-OH PhIP; |
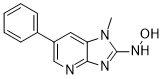
| SMILES: | CN1C(NO)=NC2=NC=C(C3=CC=CC=C3)C=C21 |
| Formula: | C13H12N4O |
| M.Wt: | 240.26 |
| Purity: | >97% |
| Publication: | 1: Vlaming ML, Teunissen SF, van de Steeg E, van Esch A, Wagenaar E, Brunsveld L, de Greef TF, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. Bcrp1;Mdr1a/b;Mrp2 combination knockout mice: altered disposition of the dietary carcinogen PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) and its genotoxic metabolites. Mol Pharmacol. 2014 Mar;85(3):520-30. doi: 10.1124/mol.113.088823. Epub 2013 Dec 13. PubMed PMID: 24334255. 2: Chen C, Ma X, Malfatti MA, Krausz KW, Kimura S, Felton JS, Idle JR, Gonzalez FJ. A comprehensive investigation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) metabolism in the mouse using a multivariate data analysis approach. Chem Res Toxicol. 2007 Mar;20(3):531-42. Epub 2007 Feb 6. PubMed PMID: 17279779; PubMed Central PMCID: PMC1850849. 3: Kulp KS, Knize MG, Fowler ND, Salmon CP, Felton JS. PhIP metabolites in human urine after consumption of well-cooked chicken. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Mar 25;802(1):143-53. PubMed PMID: 15036006. 4: Prabhu S, Lee MJ, Hu WY, Winnik B, Yang I, Buckley B, Hong JY. Determination of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP) and its metabolite 2-hydroxyamino-PhIP by liquid chromatography/electrospray ionization-ion trap mass spectrometry. Anal Biochem. 2001 Nov 15;298(2):306-13. PubMed PMID: 11700987. 5: Crofts FG, Sutter TR, Strickland PT. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998 Nov;19(11):1969-73. PubMed PMID: 9855011. 6: Crofts FG, Strickland PT, Hayes CL, Sutter TR. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human cytochrome P4501B1. Carcinogenesis. 1997 Sep;18(9):1793-8. PubMed PMID: 9328177. 7: Dragsted LO, Alexander J, Wallin H, Frandsen H, Vang O. Bioactivation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine by liver microsomes from three different rat strains. Pharmacol Toxicol. 1993 Jun;72(6):388-93. PubMed PMID: 8361949. |
| Description: | N2-OH-PhIP is a PhIP metabolite. |
| References: | 1: Vlaming ML, Teunissen SF, van de Steeg E, van Esch A, Wagenaar E, Brunsveld L, de Greef TF, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. Bcrp1;Mdr1a/b;Mrp2 combination knockout mice: altered disposition of the dietary carcinogen PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) and its genotoxic metabolites. Mol Pharmacol. 2014 Mar;85(3):520-30. doi: 10.1124/mol.113.088823. Epub 2013 Dec 13. PubMed PMID: 24334255. 2: Chen C, Ma X, Malfatti MA, Krausz KW, Kimura S, Felton JS, Idle JR, Gonzalez FJ. A comprehensive investigation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) metabolism in the mouse using a multivariate data analysis approach. Chem Res Toxicol. 2007 Mar;20(3):531-42. Epub 2007 Feb 6. PubMed PMID: 17279779; PubMed Central PMCID: PMC1850849. 3: Kulp KS, Knize MG, Fowler ND, Salmon CP, Felton JS. PhIP metabolites in human urine after consumption of well-cooked chicken. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Mar 25;802(1):143-53. PubMed PMID: 15036006. 4: Prabhu S, Lee MJ, Hu WY, Winnik B, Yang I, Buckley B, Hong JY. Determination of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine (PhIP) and its metabolite 2-hydroxyamino-PhIP by liquid chromatography/electrospray ionization-ion trap mass spectrometry. Anal Biochem. 2001 Nov 15;298(2):306-13. PubMed PMID: 11700987. 5: Crofts FG, Sutter TR, Strickland PT. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998 Nov;19(11):1969-73. PubMed PMID: 9855011. 6: Crofts FG, Strickland PT, Hayes CL, Sutter TR. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human cytochrome P4501B1. Carcinogenesis. 1997 Sep;18(9):1793-8. PubMed PMID: 9328177. 7: Dragsted LO, Alexander J, Wallin H, Frandsen H, Vang O. Bioactivation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine by liver microsomes from three different rat strains. Pharmacol Toxicol. 1993 Jun;72(6):388-93. PubMed PMID: 8361949. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.