| Cas No.: | 53910-25-1 |
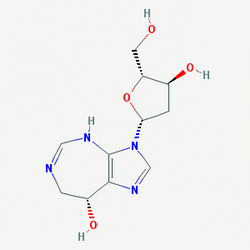
| Chemical Name: | (8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7,8-dihydro-4H-imidazo[4,5-d][1,3]diazepin-8-ol |
| Synonyms: | Deoxycoformycin |
| SMILES: | O[C@H]1C2=C(N([C@H]3O[C@H](CO)[C@@H](O)C3)C=N2)N=CNC1 |
| Formula: | C11H16N4O4 |
| M.Wt: | 268.26 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Pentostatin is an irreversible inhibitor of adenosine deaminase with Ki of 2.5 pM. |
| Target: | Ki: 2.5 pM (adenosine deaminase) |
| In Vivo: | In ECP and Pentostatin (4 mg/m2, i.v.) treatment, all dogs develop granulocytopenia with <500 granulocytes/μL from day 4. Thrombocytopenia (<20,000 platelets/μL) occurs from day 7 after HCT with nadirs of 3000 to 14000 platelets/μL[1]. Pentostatin (2 mg/kg) in combination with cordycepin (2 mg/kg) is 100% effective in the T. evansi-infected mice. There is an increase in levels of some biochemical parameters, especially on liver enzymes, which are accompanied by histological lesions in the liver and kidneys. Pentostatin individually has no curative effect on infected groups[2]. |
| Animal Administration: | All recipient dogs are conditioned for transplantation by 920 cGy TBI at 7 cGy/minute using a linear accelerator. Dogs in group A1receive ECP administered on days −2 and −1 with TBI on day 0 and dogs in group A2receive ECP on days −6 and −5, intravenous (IV) infusion of pentostatin at a dose of 4 mg/m2 on days −4 and −3, and TBI on day 0. Donor marrow cells from DLA-nonidentical donors are aspirated under general anesthesia through needles inserted into humeri and femora and stored in heparinized tissue culture medium at 4°C for no more than 6 hours. Within 4 hours of TBI, harvested marrow cells are infused IV into recipients at a median dose of 2.9 (range, 1.9 to 6.1) ×108 total nucleated cells (TNC)/kg. The day of marrow grafting is designated as day 0. In addition to marrow graft, recipients are given IV infusions of peripheral blood buffy coat cells obtained by leukapheresis from the marrow donor on days 1 and 2, at a median dose of 2.3 (range, 1.2 to 6.9) ×108 TNC/kg to ensure consistent hematopoietic engraftment. MTX, at a dose of 0.4 mg/kg intravenously is used as postgrafting immunosuppression and administered on days +1, +3, +6 and +11, then weekly thereafter until day 102. |
| References: | [1]. Bethge WA, et al. Extracorporeal photopheresis combined with pentostatin in the conditioning regimen for canine hematopoietic cell transplantation does not prevent GVHD. Bone Marrow Transplant. 2014 Sep;49(9):1198-204. [2]. Dalla Rosa L, et al. Cordycepin (3'-deoxyadenosine) pentostatin (deoxycoformycin) combination treatment of mice experimentally infected with Trypanosoma evansi. Parasitology. 2013 Apr;140(5):663-71. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.