| Cas No.: | 545380-34-5 |
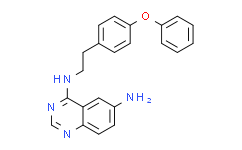
| Chemical Name: | 6-Amino-4-(4-phenoxyphenylethylamino)quinazoline |
| Synonyms: | 6-Amino-4-(4-phenoxyphenylethylamino)quinazoline;QNZ (EVP4593);4-N-[2-(4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine;EVP4593;EVP-4593;QNZ;NF-kappaB Activation Inhibitor;N4-[2-(4-Phenoxyphenyl)ethyl]-4,6-quinazolinediamine;N4-(4-phenoxyphenethyl)quinazoline-4,6-diamine;InSolution™ NF-kappaB Activation Inhibitor;C22H20N4O;EVP 4593;Curator_000005;NF-kB activation inhibitor;6-amino-4-(4-phenoxyphenylethylamino) quinazoline;QNZ(EVP45 |
| SMILES: | O(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])C([H])([H])N([H])C1C2C([H])=C(C([H])=C([H])C=2N=C([H])N=1)N([H])[H] |
| Formula: | C22H20N4O |
| M.Wt: | 356.4204 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | QNZ shows strong inhibitory effects on NF-κB transcriptional activation and TNF-α production with IC50s of 11 and 7 nM, respectively. EVP4593 is a neuroprotective inhibitor of SOC channel. |
| In Vitro: | QNZ (Compound 11q) has a suppressing effect of the NF-κB mediated-inflammatory response. QNZ inhibits edema formation dose-dependently[1]. QNZ (EVP4593) reduces the number of lysosomes/autophagosomes and store-operated channel (SOC) currents in Huntington's disease (HD). Normalization of calcium transport within neurons in response to QNZ is expect to reduce pathology manifestation. A number of lysosomes/autophagosomes are evaluated in HD and WT neurons treated with QNZ using transmission electron microscopy (TEM). Incubation with QNZ reduces the number of lysosomes/autophagosomes in HD GABAergic medium spiny (GABA MS)-like neurons (GMSLNs) by almost two-fold (from 0.41±0.04 to 0.23±0.04; p<0.05), while WT neurons are not affected. This observation is confirmed by examining lysosome content by flow cytometry (FC) analysis. The median fluorescence intensity is reduced by 34±6 % in HD GMSLNs upon QNZ treatment (p<0.05)[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.