| Cas No.: | 737789-87-6 |
| Synonyms: | TAK-385,TAK 385,TAK385 |
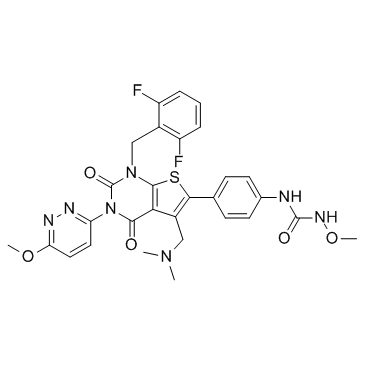
| SMILES: | O=C(NOC)NC1=CC=C(C(S2)=C(CN(C)C)C(C(N3C4=NN=C(OC)C=C4)=O)=C2N(CC5=C(F)C=CC=C5F)C3=O)C=C1 |
| Formula: | C29H27F2N7O5S |
| M.Wt: | 623.63 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Relugolix is a novel, non-peptide, orally active gonadotropin-releasing hormone (GnRH) antagonist with IC50 of 0.33 nM in the presence of 40% fetal bovine serum, TAK-385 possesses higher affinity and potent antagonistic activity compared with TAK-013. |
| Target: | .target: GnRH [1] IC50: 0.33 nM [1] |
| In Vivo: | In female knock-in mice, twice-daily oral administration of TAK-385 (100mg/kg) induces constant diestrous phases within the first week, decreases the uterus weight to ovariectomized levels and downregulated GnRH receptor mRNA in the pituitary after 4 weeks.[2] |
| References: | [1]. MacLean DB et al. Medical Castration Using the Investigational Oral GnRH Antagonist TAK-385 (Relugolix): Phase 1 Study in Healthy Males. J Clin Endocrinol Metab. 2015 Dec;100(12):4579-87. [2]. Nakata D et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Ph |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.