| Cas No.: | 415697-08-4 |
| Chemical Name: | SN 6 |
| Synonyms: | 4-Thiazolidinecarboxylicacid, 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-, ethyl ester;SN 6;2-[[4-[(4-NITROPHENYL)METHOXY]PHENYL]METHYL]-4-THIAZOLIDINECARBOXYLIC ACID ETHYL ESTER;ethyl 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-1,3-thiazolidine-4-carboxylate;SN-6;HMS3268P11;2-[4-(4-Nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester;4-Thiazolidinecarboxylic acid, 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-, ethyl ester;HY-107658;HY-107658;HMS3677K09;HMS3677K09;CS-0029062;CS-0029062;BRD-A14316475-001-01-5;BRD-A14316475-001-01-5;Ethyl 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-1,3-thiazolidine-4-carboxylate;Ethyl 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-1,3-thiazolidine-4-carboxylate;415697-08-4;415697-08-4;NCGC00092323-01;SN-6, >=98% (HPLC);NCGC00092323-01;AKOS024456975;SN-6, >=98% (HPLC);N16889;AKOS024456975;HMS3413K09;CHEMBL1318259;N16889;Ethyl 2-(4-((4-nitrobenzyl)oxy)benzyl)thiazolidine-4-carboxylate;DTXSID70436978;HMS3413K09;SN 6; SN6;CHEMBL1318259;BCP32885;Ethyl 2-(4-((4-nitrobenzyl)oxy)benzyl)thiazolidine-4-carboxylate;BS-16804;DTXSID70436978;SN 6; SN6;BCP32885;BS-16804 |
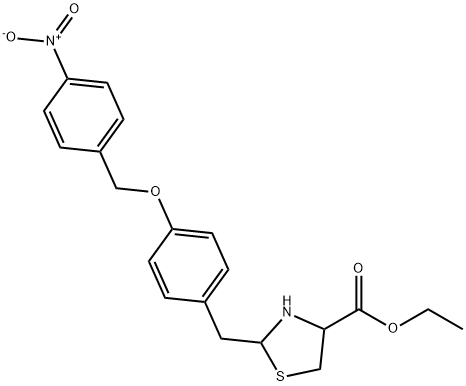
| SMILES: | CCOC(C(CS1)NC1CC2=CC=C(OCC3=CC=C([N+]([O-])=O)C=C3)C=C2)=O |
| Formula: | C20H22N2O5S |
| M.Wt: | 402.46408 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | SN 6 is a selective Na+/Ca2+ exchanger (NCX) inhibitor, and inhibits 45Ca2+ uptake by NCX1, NCX2, and NCX3, with IC50s of 2.9, 16, and 8.6 μM, respectively. |
| Target: | IC50:2.9 μM (NCX1), 16 μM (NCX2), 8.6 μM (NCX3)[1] |
| In Vitro: | SN 6 is a selective Na+/Ca2+ exchanger inhibitor, which inhibits the initial rate of 45Ca2+ uptake into NCX1, NCX2, and NCX3 transfectants with IC50 values of 2.9 ± 0.12, 16 ± 1.1, and 8.6 ± 0.27 μM. SN 6 (up to 30 μM) also less potently inhibits muscarinic acetylcholine receptor, with a higher IC50 of 18 μM. SN 6 (0.3-30 μM) completely inhibits the initial rate of Na+i-dependent 45Ca2+ uptake into Na+-loaded sarcolemmal vesicles in a dose dependent manner (IC50, 5.3 ± 0.37 μM). SN 6 (0.3-10 μM) dose-dependently protects against the hypoxia/reoxygenation-induced LDH release in parental LLC-PK1 cells and NCX1 transfectants but not in K229Q transfectants[1]. SN 6 (1-30 μM) suppresses the bidirectional outward and inward INCX in a concentration-dependent manner, with IC50 values of 2.3 μM and 1.9 μM, respectively. SN 6 also inhibits bidirectional current (INCX) in a [Na+]i concentration-dependent manner, with IC50 values of 3.4 μM, 2.3 μM, and 1.1 μM at 10 mM, 20 mM, and 30 mM [Na+]i, respectively[2]. SN 6 inhibits hypoxia/reoxygenation-induced LDH release with an IC50 value of 0.63 ± 0.15 μM in NCX1 transfectants[3]. |
| Cell Assay: | Na+i-dependent 45Ca2+ uptake into cells expressing the wild-type or mutated exchangers are assayed. In brief, confluent transfectants in 24-well dishes are loaded with Na+ by incubation at 37°C for 40 min in 0.5 mL of balanced salt solution (BSS) (10 mM HEPES/Tris, pH 7.4, 146 mM NaCl, 4 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 10 mM glucose, and 0.1% bovine serum albumin) containing 1 mM ouabain and 10 μM monensin. 45Ca2+ uptake is then initiated by switching the medium to Na+-free BSS (replacing NaCl with equimolar choline chloride) or to normal BSS, both of which contain 0.1 mM 45CaCl2 (370 kBq/mL) and 1 mM ouabain. After a 30-s incubation, 45Ca2+ uptake is terminated by washing cells four times with an ice-cold solution containing 10 mM HEPES/Tris, pH 7.4, 120 mM choline chloride, and 10 mM LaCl3. Cells are then solubilized with 0.1 N NaOH, and aliquots are taken for determination of radioactivity and protein. When present, SN 6 and KB-R7943 are included in the medium 15 min before the start of 45Ca2+ uptake[1]. |
| References: | [1]. Iwamoto T, et al. The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol Pharmacol. 2004 Jul;66(1):45-55. [2]. Niu CF, et al. Electrophysiological effects of SN-6, a novel Na+/Ca2+ exchange inhibitor on membrane currents in guinea pig ventricular myocytes. Ann N Y Acad Sci. 2007 Mar;1099:534-9. [3]. Kita S, et al. Inhibitory mechanism of SN-6, a novel benzyloxyphenyl Na+/Ca2+ exchange inhibitor. Ann N Y Acad Sci. 2007 Mar;1099:529-33. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.