| Cas No.: | 1702809-17-3 |
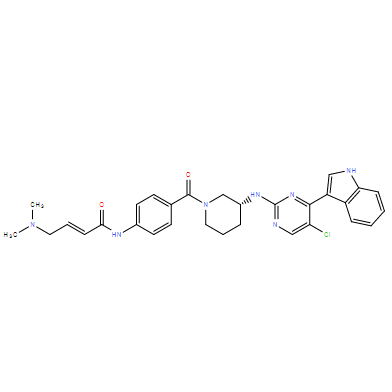
| Chemical Name: | (R,E)-N-(4-(3-((5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl)amino)piperidine-1-carbonyl)phenyl)-4-(dimethylamino)but-2-enamide |
| Synonyms: | THZ531; THZ-531; THZ 531 |
| SMILES: | O=C(NC1=CC=C(C(N2C[C@H](NC3=NC=C(Cl)C(C4=CNC5=C4C=CC=C5)=N3)CCC2)=O)C=C1)/C=C/CN(C)C |
| Formula: | C30H32ClN7O2 |
| M.Wt: | 557.23 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | THZ531 is a covalent inhibitor of both CDK12 and CDK13 with IC50s of 158 nM and 69 nM, respectively. |
| In Vitro: | The results from Kinase assays demonstrate that THZ531 potently inhibits CDK12 and CDK13 with IC50s of 158 nM and 69 nM, respectively; whereas inhibition of CDK7 and CDK9 is more than 50-fold weaker with IC50s of 8.5 and 10.5 µM, respectively. THZ531 treatment leads to a dramatic and irreversible decrease in Jurkat cell proliferation with an IC50 of 50 nM. FACS cell cycle analysis following treatment with escalating doses of THZ531 displays a dose and time-dependent increase in the number of cells exhibiting sub-G1 content. At 50 nM THZ531, no increase in the percentage of apoptotic cells is observed over DMSO control for the time course of the experiment. Higher doses of THZ531 leads to pronounced Annexin V signal with 30 to 40% annexin V-positively stained cells by 72 hrs. A dramatic reduction in elongating Pol II following THZ531 treatment is also observed[1]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.