| Cas No.: | 577778-58-6 |
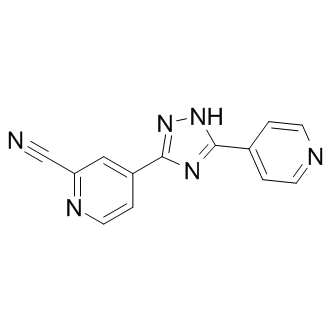
| Chemical Name: | 4-(5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl)picolinonitrile |
| Synonyms: | FYX-051; FYX051; FYX 051; Topiroxostat. trade names Topiloric, Uriadec. |
| SMILES: | N1C(C2C=CN=C(C#N)C=2)=NC(C2C=CN=CC=2)=N1 |
| Formula: | C13H8N6 |
| M.Wt: | 248.24 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks4°C in DMSO,6 months-80°C in DMSO |
| Description: | Topiroxostat(FYX-051) is a novel and potent xanthine oxidoreductase (XOR) inhibitor with IC50 value of 5.3 nM. |
| Target: | IC50 value: 5.3 nM [1] Target: xanthine oxidoreductase |
| In Vivo: | FYX-051 exhibited a weak CYP3A4-inhibitory activity (18.6%); its Cmax and bioavailability were as high as 4.62 μg/mL (3 mg/kg) and 69.6%, respectively. Moreover, the t1/2 value of 39 was greater (19.7 h) than that of compound 2 (0.97 h) [1]. In the mechanistic study by 52-week oral treatment with topiroxostat at 3 mg/kg to F344 male rats, with and without citrate, simple and papillary transitional cell hyperplasias of the urinary bladder epithelium were observed in 5/17 in the topiroxostat-alone treatment group, along with xanthine-induced nephropathy, in contrast to neither xanthine crystals nor lesions in urinary organs by co-treatment group with citrate [2]. |
| In Vitro: | Steady-state kinetics study showed that FYX-051 initially behaved as a competitive-type inhibitor with a K(i) value of 5.7 × 10(-9) M, then after a few minutes it formed a tight complex with XOR via a Mo-oxygen-carbon atom covalent linkage, as reported previously [3] |
| References: | [1]. Sato T, et al. Discovery of 3-(2-cyano-4-pyridyl)-5-(4-pyridyl)-1,2,4-triazole, FYX-051 - a xanthine oxidoreductase inhibitor for the treatment of hyperuricemia [corrected]. Bioorg Med Chem Lett. 2009 Nov 1;19(21):6225-9. [2]. Shimo T, et al. Xanthine crystals induced by topiroxostat, a xanthine oxidoreductase inhibitor, in rats, cause transitional cell tumors. Arch Toxicol. 2014 Jan 22. [3]. Matsumoto K, et al. FYX-051: a novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther. 2011 Jan;336(1):95-103. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.