| Cas No.: | 175865-59-5 |
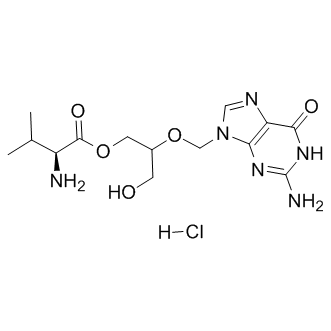
| Chemical Name: | [2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]-3-hydroxypropyl] (2S)-2-amino-3-methylbutanoate hydrochloride |
| Synonyms: | RS 079070-194, RO 107-9070/194, RS-79070-194, RS-079070-194, ganciclovir L-valyl ester, Valganciclovir HCl, Cymeval, Valcyt, Valixa, Darilin, Rovalcyte, Patheon |
| SMILES: | O=C1C2=C(N(COC(CO)COC([C@H](C(C)C)N)=O)C=N2)N=C(N)N1.Cl |
| Formula: | C14H23ClN6O5 |
| M.Wt: | 390.825 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Valganciclovir (hydrochloride), the L-valyl ester of ganciclovir, is actually a prodrug for ganciclovir. Valganciclovir is an antiviral medication used to treat cytomegalovirus infections. in vitro: In cell culture model systems using Caco-2 cells for PEPT1 and SKPT cells for PEPT2, valganciclovir inhibited glycylsarcosine transport mediated by PEPT1 and PEPT2 with K(i) values (inhibition constant) of 1.68+/-0.30 and 0.043+/- 0.005 mM, respectively. The inhibition by valganciclovir was competitive in both cases. in vivo: 37 patients were enrolled; 19 patients received treatment with VGV and 18 patients received treatment with GCV. The VGV was not inferior in efficacy to GCV as pre-emptive therapy, with rates of viral clearance at 28 days of 89.5% and 83%, respectively (P-value for non-inferiority = 0.030). Toxicities were similar between the 2 arms. No patients developed CMV disease. Patients being treated with an alemtuzumab-containing regimen received prophylaxis with either valaciclovir 500 mg orally daily orvalganciclovir 450 mg orally twice daily. None of the 20 patients randomized to valganciclovir experienced CMV reactivation (P = .004). |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.