| Cas No.: | 1454885-45-0 |
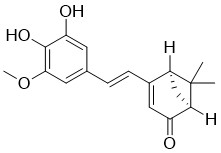
| Chemical Name: | (1S,5R)-4-[(E)-2-(3,4-Dihydroxy-5-methoxyphenyl)ethenyl]-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one |
| Synonyms: | (1S,5R)-4-[(E)-2-(3,4-Dihydroxy-5-methoxyphenyl)ethenyl]-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one |
| SMILES: | O=C1C([H])=C(/C(/[H])=C(\[H])/C2=C([H])C(=C(C(=C2[H])OC([H])([H])[H])O[H])O[H])[C@]2([H])C([H])([H])[C@@]1([H])C2(C([H])([H])[H])C([H])([H])[H] |
| Formula: | C18H20O4 |
| M.Wt: | 300.3490 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | [1]. Mander S, et al. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci Rep. 2019 Apr 29;9(1):6595. [2]. Pahk K, et al. SP-8356, a Novel Inhibitor of CD147-Cyclophilin A Interactions, Reduces Plaque Progression and Stabilizes Vulnerable Plaques in apoE-Deficient Mice. Int J Mol Sci. 2019 Dec 21;21(1). pii: E95. [3]. Pahk K, et al. A novel CD147 inhibitor, SP-8356, reduces neointimal hyperplasia and arterial stiffness in a rat model of partial carotid artery ligation. J Transl Med. 2019 Aug 20;17(1):274. |
| Description: | SP-8356, an anti-inflammatory synthetic verbenone derivative, is a potent, orally active cluster of differentiation 147 (CD147) inhibitor. SP-8356 inhibits CD147-cyclophilin A (CypA) interaction and reduces MMP-9 activity. SP-8356 exerts anti-breast cancer effects by inhibiting NF-κB signaling. Anti-atherosclerotic effects[1][2][3]. |
| In Vivo: | SP-8356 (10 mg/kg; i.p.; every three days until the 42nd day) inhibits tumor growth in a xenograft mouse model[1]. SP-8356 (50 mg/kg; p.o.; daily one day after carotid artery ligation for three weeks) prevents the formation of plaque and attenuates its vulnerability[2]. Animal Model: Five-week-old female NOD/SCID mice (MDA-MB231 xenografts)[1] Dosage: 10 mg/kg Administration: I.p.; every three days until the 42nd day Result: The tumor volumes of mice were significantly lower. Animal Model: Male ApoE KO mice (7 weeks old, 20 g body weight)[2] Dosage: 50 mg/kg Administration: P.o.; daily one day after carotid artery ligation for three weeks Result: Induced a significant reduction in plaque size and plaque/media ratiowith decreased lipid and necrotic core areas. |
| In Vitro: | SP-8356 (5-10 μM; 48 hours) inhibits growth of various types of breast cancer cell lines in a time- and dose-dependent manner[1]. SP-8356 (10 μM; 48 hours) increases percentage of MDA-MB231 cells in S phase and decreases casapas-3 and cleaved PARP[1]. Cell Proliferation Assay[1] Cell Line: MDA-MB231, MDA-MB453, 4T1, and MCF-7 cells Concentration: 5, 10 μM Incubation Time: 48 hours Result: Inhibited growth of various types of breast cancer cell lines in a time- and dose-dependent manner. Cell Cycle Analysis[1] Cell Line: MDA-MB231 cells Concentration: 1, 5, 10 μM Incubation Time: 48 hours Result: Increased percentage of MDA-MB231 cells in S phase at 10 μM. Western Blot Analysis[1] Cell Line: MDA-MB231 cells Concentration: 10 μM Incubation Time: 48 hours Result: Decrease of casapas-3 and cleaved PARP were observed. |
| References: | [1]. Mander S, et al. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci Rep. 2019 Apr 29;9(1):6595. [2]. Pahk K, et al. SP-8356, a Novel Inhibitor of CD147-Cyclophilin A Interactions, Reduces Plaque Progression and Stabilizes Vulnerable Plaques in apoE-Deficient Mice. Int J Mol Sci. 2019 Dec 21;21(1). pii: E95. [3]. Pahk K, et al. A novel CD147 inhibitor, SP-8356, reduces neointimal hyperplasia and arterial stiffness in a rat model of partial carotid artery ligation. J Transl Med. 2019 Aug 20;17(1):274. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.