| Cas No.: | 2607138-82-7 |
| Chemical Name: | ART812 |
| Synonyms: | 2-Pyrrolidinecarboxamide, N-(3-chloro-4-fluorophenyl)-3,4-dihydroxy-N-methyl-1-[6-methyl-4-(trifluoromethyl)-2-pyridinyl]-5-oxo-, (2S,3S,4S)-;(2S,3S,4S)-N-(3-chloro-4-fluorophenyl)-3,4-dihydroxy-N-methyl-1-(6-methyl-4-(trifluoromethyl)pyridin-2-yl)-5-oxopyrrolidine-2-carboxamide;ART812 |
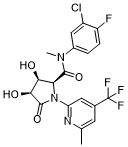
| SMILES: | N1(C2=NC(C)=CC(C(F)(F)F)=C2)C(=O)[C@@H](O)[C@@H](O)[C@H]1C(N(C1=CC=C(F)C(Cl)=C1)C)=O |
| Formula: | C19H16ClF4N3O4 |
| M.Wt: | 461.79 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | ART812 is a DNA polymerase Polθ inhibitor with an IC50 value of 7.6 nM. ART812 has an IC50 value of 240 nM for cell based microhomology-mediated end joining (MMEJ)[1][2]. |
| Target: | Polθ:7.6 nM (IC50) |
| In Vivo: | ART812 (100 mg/kg; p.o. daily for 76 days) shows significant tumour inhibition in rats bearing established MDA-MB-436 BRCA1/SHLD2 defective tumours (volume 250-350 mm3)[1]. |
| In Vitro: | ART812 (0-40 μM) elicits Polθ inhibitor sensitivity in MDA-MB-436 SHLD2 knockout cells[1]. |
| References: | [1]. Zatreanu D, et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat Commun. 2021 Jun 17;12(1):3636. [2]. Peter BLENCOWE, et al. Preparation of heterocyclic compounds for use in the treatment of cancer. WO2021028643 A1. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.