| Cas No.: | 1108743-60-7 |
| Chemical Name: | Entrectinib |
| Synonyms: | N-(5-(3,5-difluorobenzyl)-1H-indazol-3-yl)-4-(4-methylpiperazin-1-yl)-2-(tetrahydro-2H-pyran-4-ylamino)benzamide;Entrectinib;NMS-E628;RXDX-101;N-[5-[(3,5-Difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]benzamide;RXDX 101;Entrectinib(RXDX-101);L5ORF0AN1I;Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-;Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-;N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methylpiperazin-1-yl)-2-(oxan-4-ylamino)benzamide;Entrectinib [USAN:INN];Rozlytrek;YMX;Entrectinib,;N-{5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl}-4-(4-methylpiperazin-1-yl)-2-[(oxan-4-yl)amino]benzamide;N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3- |
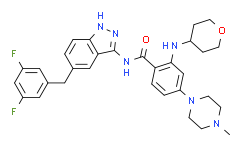
| SMILES: | FC1C([H])=C(C([H])=C(C=1[H])C([H])([H])C1C([H])=C([H])C2=C(C=1[H])C(=NN2[H])N([H])C(C1C([H])=C([H])C(=C([H])C=1N([H])C1([H])C([H])([H])C([H])([H])OC([H])([H])C1([H])[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)F |
| Formula: | C31H34F2N6O2 |
| M.Wt: | 560.6375 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Entrectinib is a potent and orally available Trk, ROS1, and ALK inhibitor; inhibits TrkA, TrkB, TrkC, ROS1 and ALK with IC50 values of 1, 3, 5, 12 and 7 nM, respectively. |
| In Vivo: | Oral administration of entrectinib to tumor-bearing mice induces regression in relevant human xenograft tumors, including the TRKA-dependent colorectal carcinoma KM12, ROS1-driven tumors, and several ALK-dependent models of different tissue origins, including a model of brain-localized lung cancer metastasis[1]. Single agent therapy results in significant tumor growth inhibition in animals treated with entrectinib compared to control animals[2]. |
| In Vitro: | Entrectinib is found to be exquisitely active in inhibiting the proliferation of a limited number of cell lines: the TRKA-driven colorectal carcinoma cell line KM12 (IC50 of 17 nM), the ALK-dependent ALCL cell lines SU-DHL-1, Karpas-299, SUP-M2 and SR-786 (IC50 of 20, 31, 41, and 81 nM, respectively), the ALK-dependent NSCLC cell line NCI-H2228 (IC50 of 68 nM) and the FLT3-dependent AML cell line MV-4-11 (IC50 of 81 nM). Entrectinib potently blocks proliferation of Ba/F3-TEL-TRKB (IC50 of 2.9 nM), Ba/F3-TEL-TRKC (IC50 of 3.3 nM), and Ba/F3-TEL-ROS1 (IC50 of 5.3 nM) cells, with a high degree of selectivity versus parental Ba/F3 cells or those transformed by nontargeted kinases such as ABL and RET, which are inhibited with IC50s in the range of 2 to 3 μM[1]. Entrectinib significantly inhibits the growth of TrkB-expressing NB cells in vitro, and it significantly enhances the growth inhibition of Irino-TMZ when used in combination[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.