| Cas No.: | 142880-36-2 |
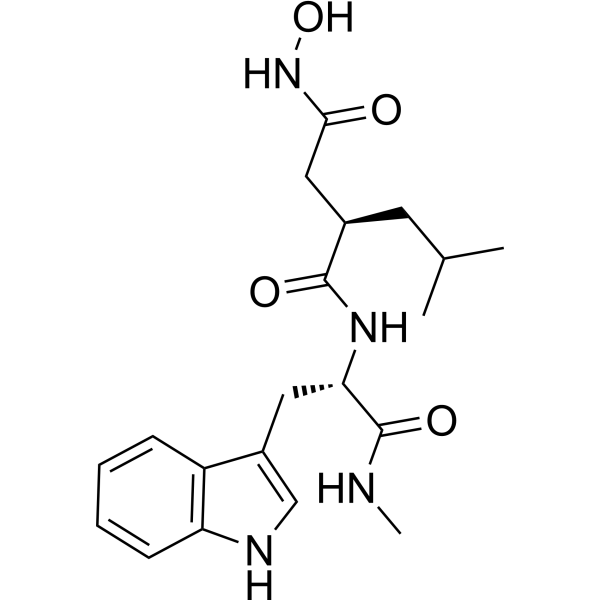
| Chemical Name: | Ilomastat |
| Synonyms: | Butanediamide,N4-hydroxy-N1-[(1S)-1-(1H-indol-3-ylmethyl)-2-(methylamino)-2-oxoethyl]-2-(2-methylpropyl)-,(2R)-;Butanediamide,N4-hydroxy-N1-[(1S)-1-(1H-indol-3-ylmethyl)-2-(methylamino)-2-oxoethyl]-2-(2-met...;GM 6001 (Ilomastat,Galardin);Ilomastat;Ilomastat (GM6001, Galardin);(2R)-N~4~-hydroxy-N~1~-[(2S)-3-(1H-indol-3-yl)-1-(methylamino)-1-oxopropan-2-yl]-2-(2-methylpropyl)butanediamide;(r)-n4-hydroxy-n1-[(s)-2-(1h-indol-3-yl)-1-methylcarbamoly-ethyl]-2-isobutyl-succinamide;(R)-N4-Hydroxy-N1-[(S)-2-(1H-indol-3-yl)-1-methylcarbamoyl-ethyl]-2-isobutyl-succinamide;Galardin;galardin tm;Galardin, GM6001, Ilomastat;GM-6001;GM6001;CS 610;(2R)-N4-Hydroxy-N1-[(1S)-1-(1H-indol-3-ylmethyl)-2-(methylamino)-2-oxoethyl]-2-(2-methylpropyl)-butanediamide;GM 6001;Illomastat;I0403ML141;N-[(2R)-2-(Hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan Methylamide;(R)-N(sup 1)-Hydroxy-N-((S)-2-indol-3-yl-1-(methylcarbamoyl)ethyl)-2-isobutylsuccinamide;(S-(R*,S*))-N(sup 4)-Hydroxy-N(sup 1)-(1H-indol-3-ylmethyl)-2-(methylam |
| SMILES: | O=C([C@@]([H])(C([H])([H])C(N([H])O[H])=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])[C@]([H])(C(N([H])C([H])([H])[H])=O)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 |
| Formula: | C20H28N4O4 |
| M.Wt: | 388.4607 |
| Purity: | 98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Ilomastat (Galardin; GM6001) is a broad spectrum matrix metalloprotease (MMP) inhibitor, with Ki of 0.4 nM, 0.5 nM, 27 nM, 3.7 nM, 0.1 nM, 0.2 nM, 3.6 nM, 13.4 nM, 0.36 nM for MMP-1/2/3/7/8/9/12/14/26, respectively. |
| In Vivo: | Ilomastat (GM6001) (400 μg/mL) prevents corneal ulceration after severe alkali injury[2]. Ilomastat (GM6001) significantly inhibits intimal hyperplasia and intimalcollagen content, and it increases lumen area in stented arteries without effects on proliferation rates in rabbit model after stenting[3]. |
| In Vitro: | Ilomastat (GM6001) inhibits human skin fibroblast collagenase with Ki of 0.4 nM when assayed with a synthetic thio ester substrate at pH 6.5, with 50-fold selectivity over two bacterial enzymes, thermolysin and Pseudomonas aeruginosa elastase[1]. Ilomastat (0.1 nM-10 nM) inhibits gelatinase A and gelatinase B produced by T-cells, and thus inhibits T-cell homing[4]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.