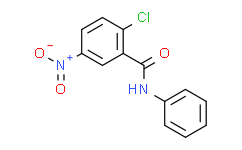
| Cas No.: | 22978-25-2 |
| Chemical Name: | 2-Chloro-5-nitro-N-phenylbenzamide |
| Synonyms: | Benzamide,2-chloro-5-nitro-N-phenyl-;GW9662;2-Chloro-5-nitrobenzanilide;2-Chloro-5-nitro-N-4-phenylbenzamide;GW 9662 (GW-9662);2-chloro-5-nitro-N-phenylbenzamide;GW-9662;GW 9662;MLS001056751;2-Chloro-5-nitro-N-phenyl-benzamide;benzamide, 2-chloro-5-nitro-N-phenyl-;SMR000326735;(2-chloro-5-nitrophenyl)-N-benzamide;Spectrum5_001989;DSSTox_RID_79570;DSSTox_CID_20723;DSSTox_GSID_40723;Lopac0_000798;KBioGR_000361;KBioSS_000361;BSPBio_001021;GTP |
| SMILES: | ClC1C([H])=C([H])C(=C([H])C=1C(N([H])C1C([H])=C([H])C([H])=C([H])C=1[H])=O)[N+](=O)[O-] |
| Formula: | C13H9ClN2O3 |
| M.Wt: | 276.6752 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | GW9662 is a potent and selective PPARγ antagonist with an IC50 of 3.3 nM, showing 10 and 1000-fold selectivity over PPARα and PPARδ, respectively. |
| In Vivo: | Bone marrow (BM) nucleated cell counts in both BADGE- and GW9662(1 mg/kg, i.p.)-treated mice are significantly higher than counts in the aplastic anemia (AA) group[3]. GW9662 (1 mg/kg, i.p.) largely attenuates the renoprotective effects of Lipopolysaccharide (LPS) in the rat[4]. |
| In Vitro: | GW9662 inhibits radioligand binding to PPARγ, PPARα, and PPARδ with pIC50s of 8.48±0.27 (IC50=3.3 nM; n=10), 7.49±0.17 (IC50=32 nM; n=9), and 5.69±0.17 (IC50=2000 nM; n=3), respectively. GW9662 has nanomolar IC50 versus PPARγ and is 10- and 600-fold less potent in binding experiments using PPARα and PPARδ, respectively. In cell-based reporter assays, GW9662 is a potent and selective antagonist of full-length PPARγ[1]. Co-treatment with both 50 μM Rosiglitazone and 10 μM GW9662 results in statistically lower viable cell numbers after 7 days when compared to treatment with either 50 μM rosiglitazone (P=0.001) or 10 μM GW9662 (P=0.01) alone[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.