| Cas No.: | 1127442-82-3 |
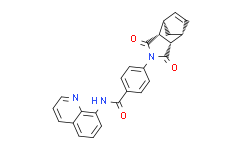
| Chemical Name: | 4-(3,5-Dioxo-4-azatricyclo[5.2.1.02,6]dec-8-en-4-yl)-N-quinolin-8-ylbenzamide |
| Synonyms: | IWR-1;IWR-1-endo;endo-IWR 1;IWP-1-endo;IWR-1 endo (IWR-1-endo, endo IWR-1 );IWR1;IWR1-endo;ChemDiv1_005302;Oprea1_443063;Oprea1_037138;HMS602A22;4-(3,5-Dioxo-4-azatricyclo[5.2.1.02,6]dec-8-en-4-yl)-N-quinolin-8-ylbenzamide |
| SMILES: | O=C1C2([H])C3([H])C([H])=C([H])C([H])(C3([H])[H])C2([H])C(N1C1C([H])=C([H])C(C(N([H])C2=C([H])C([H])=C([H])C3C([H])=C([H])C([H])=NC2=3)=O)=C([H])C=1[H])=O |
| Formula: | C25H19N3O3 |
| M.Wt: | 409.4367 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | IWR-1 is a tankyrase inhibitor which inhibits Wnt/β-catenin signaling pathway. |
| In Vitro: | Both IWR-1 and XAV939 act as reversible Wnt pathway inhibitors and exhibit similar pharmacological effects in vitro. IWR-1 exerts its effect via interaction with Axin, while XAV939 binds TNKS directly[1]. IWR-1 (10 μM) induces stabilization of β-catenin disruption complex. IWR-1 (10 μM) is added to the medium together with MIF, the size of cell colonies is extremely decreased, and that indicates the promoting effect of MIF on NSPC proliferation is inhibited by IWR-1 in any MIF concentration group. 2, 5 and 10 μM of IWR-1 significantly inhibits the proliferation of NSPC dose-dependently. IWR-1 inhibites the promoting effect of MIF on NSPC differentiation to neuron lineage[2]. IWR-1 treatment in the presence of maximal stimulatory dose of FSH (0.5 ng/mL) results in a dose dependent inhibition of the stimulatory effect of FSH with > 75% inhibition observed at the maximal inhibitory dose of IWR-1 (1 µM). IWR-1 treatment partially reverses the FSH-induced inhibition of granulosa cell CARTPT mRNA expression[3]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.