| Cas No.: | 1094873-14-9 |
| Chemical Name: | JNJ-31020028 |
| Synonyms: | DG 051 (free base);N-[4-[4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl]-3-fluorophenyl]-2-pyridin-3-ylbenzamide;UNII-73F8XED6YP;N,N-Diethyl-4-[2-fluoro-4-[[2-(3-pyridinyl)benzoyl]amino]phenyl]-alpha-phenyl-1-piperazineacetamide;JNJ-31020028;JNJ 31020028;JNJ31020028;OVUNRYUVDVWTTE-UHFFFAOYSA-N;N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide;BCP14600;BDBM50352371;BC600690;N-(4-(4-((N,N-Diethylcarbamoyl)(phenyl)methyl)piperazin-1-yl)-3-fluorophenyl)-2-(pyridin-;s6416;N-(4-(4-((N,N-Diethylcarbamoyl)(phenyl)methyl)piperazin-1-yl)-3-fluorophenyl)-2-(pyridin-3-yl)benzamide;AK499432;1-Piperazineacetamide, N,N-diethyl-4-(2-fluoro-4-((2-(3-pyridinyl)benzoyl)amino)phenyl)-alpha-phenyl-;Q27266173;N-{4-[4-(Diethylcarbamoyl-phenyl-methyl)-piperazin-1-yl]-3-fluoro-phenyl}-2-py |
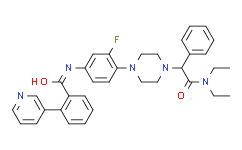
| SMILES: | FC1C([H])=C(C([H])=C([H])C=1N1C([H])([H])C([H])([H])N(C([H])(C(N(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])[H])=O)C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H])N([H])C(C1=C([H])C([H])=C([H])C([H])=C1C1=C([H])N=C([H])C([H])=C1[H])=O |
| Formula: | C34H36FN5O2 |
| M.Wt: | 565.6 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | JNJ-31020028 is a selective brain penetrant antagonist of neuropeptide Y2 receptor with high affinity(pIC50=8.07, human; pIC50=8.22 rat); >100-fold selective versus human Y1/Y4/Y5 receptors.IC50 value: 8.07/8.22(human/rat pIC50) [1]Target: Y2 receptor antagonistin vitro: JNJ-31020028 was demonstrated to be an antagonist (pK(B) = 8.04 +/- 0.13) in functional assays [1].in vivo: JNJ-31020028 occupied Y(2) receptor binding sites (approximately 90% at 10 mg/kg) after subcutaneous administration in rats [1]. Neither systemic (0, 15, 30, and 40 mg/kg, subcutaneously [s.c.]) nor intracerebroventricular (0.0, 0.3, and 1.0 nmol/rat) administration of JNJ-31020028 affected alcohol-reinforced lever pressing or relapse to alcohol seeking behavior following stress exposure. JNJ-31020028 (15 mg/kg, s.c.) did reverse the anxiogenic effects of withdrawal from a single bolus dose of alcohol on the elevated plus-maze, confirming the anxiolytic-like properties of NPY Y2 antagonism [2]. Chronic administration of JNJ-31020028 induced a decrease in immobility time in the forced swim test in OBX while had no effect in control animals [3]. |
| References: | [1]. Shoblock JR, et al. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y [2]. Cippitelli A, et al. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011 Sep;45(6):567-76. [3]. Morales-Medina JC, et al. Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat. Neuropeptides. 2012 Dec;46(6):329-34. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.