| Cas No.: | 875787-07-8 |
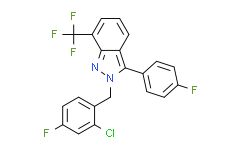
| Chemical Name: | 2-(2-Chloro-4-fluorobenzyl)-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole |
| Synonyms: | 2H-Indazole, 2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)-;2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)indazole;LXR 623(WAY 252623);LXR623;WAY252623;WAY-252623;2-[(2-Chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole;LXR-623;2-(2-Chloro-4-fluorobenzyl)-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole;WAY 252623;LXR 623;4W82FEU838;2H-Indazole, 2-((2-chloro-4-fluorophenyl)methyl)-3-(4-fluorophenyl)-7-(trifluoromethyl)-;LX623;C21H12ClF5N2 |
| SMILES: | ClC1C([H])=C(C([H])=C([H])C=1C([H])([H])N1C(C2C([H])=C([H])C(=C([H])C=2[H])F)=C2C([H])=C([H])C([H])=C(C(F)(F)F)C2=N1)F |
| Formula: | C21H12ClF5N2 |
| M.Wt: | 422.7784 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LXR-623 is a brain-penetrant partial LXRα and full LXRβ agonist, with IC50s of 24 nM and 179 nM, respectively. |
| In Vivo: | LXR-623 (400 mg/kg, p.o.) crosses the blood-brain barrier, induces target gene expression, and achieves therapeutic levels in GBM cells in the brain with minimal activity in the periphery. LXR-623 inhibits tumor growth, promotes tumor cell death, and prolongs the survival of mice bearing intracranial patient-derived GBMs[1]. LXR-623 (1.5, 5 mg/kg/day) significantly reduces progression of atherosclerosis in animals compared with the placebo group[2]. WAY-252623 (15 and 50 mg/kg) results in a significant reduction of atherosclerosis in a dose-dependent manner. WAY-252623 (20, 60, and 120 mg/kg/day, p.o.) displays neutral lipid effects in this CETP-expressing Syrian hamster[3]. Moreover, LXR-623 (50 mg/kg) induces gene expression in rodent peripheral blood cells in rat. LXR-623 (0, 15 and 50 mg/kg) dose-dependently upregulates transcription of ABCA1 and ABCG1 in monkey whole blood cells proportional to dose[4]. |
| In Vitro: | LXR-623 potently kills U87EGFRvIII and GBM39 cells in vitro while completely sparing NHAs. LXR-623 also increases ABCA1 protein and decreases LDLR protein levels in all three cell lines. LXR-623 suppresses LDLR expression, increases expression of the ABCA1 efflux transporter, and induces substantial cell death in all of the GBM samples tested. LXR-623 (5 μM) also induces GBM cell death through activation of LXRβ[1]. LXR-623 treatment of human PBMC in vitro significantly increases transcription of ABCA1 and ABCG1[4]. |
| Animal Administration: | Five-week-old female athymic nu/nu mice are used in the experiment. A total of 1×105 U87EGFRvIII IRFP720 or GBM39 IRFP720 cells in 5 μL of PBS is intracranially injected into the mouse brain. Tumors are allowed to establish over the course of 7-10 days and engraftment of tumors is quantitatively confirmed via FMT signal intensity. Tumor growth is monitored using an FMT 2500 fluorescence tomography system. For drug treatment studies, vehicle (0.5% methylcellulose, 2% Tween 80 in water) or LXR-623 (400 mg/kg) resuspended in vehicle are administered to mice via oral gavage daily starting at day 7 postinjection. |
| References: | [1]. Villa GR, et al. An LXR-Cholesterol Axis Creates a Metabolic Co-Dependency for Brain Cancers. Cancer Cell. 2016 Nov 14;30(5):683-693. [2]. Giannarelli C, et al. Synergistic effect of liver X receptor activation and simvastatin on plaque regression and stabilization: an magnetic resonance imaging study in a model of advanced atherosclerosis. Eur Heart J. 2012 Jan;33(2):264-73. [3]. Quinet EM, et al. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res. 2009 Dec;50(12):2358-70. [4]. DiBlasio-Smith EA, et al. Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells. J Transl Med. 2008 Oct 16;6:59. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.