| Cas No.: | 1819363-80-8 |
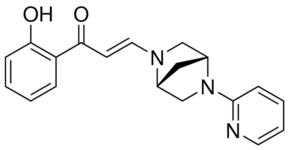
| Chemical Name: | (E)-1-(2-Hydroxyphenyl)-3-((1R,4R)-5-(pyridin-2-yl)-2,5-diazabicyclo[2.2.1]heptan-2-yl)prop-2-en-1-one |
| Synonyms: | PFI3, PFI 3 |
| SMILES: | C1[C@@H]2CN([C@H]1CN2C3=CC=CC=N3)/C=C/C(=O)C4=CC=CC=C4O |
| Formula: | C19H19N3O2 |
| M.Wt: | 321.37 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | PFI-3 is a selective, potent and cell-permeable SMARCA2/4 bromodomain inhibitor with a Kd of 89 nM. |
| In Vitro: | PFI-3 is a potent, cell-permeable probe capable of displacing ectopically expressed, GFP-tagged SMARCA2-bromodomain from chromatin. PFI-3 binds avidly to both SMARCA2 and SMARCA4 bromodomains (BROMOScan Kd's between 55 and 110 nM) consistent with the binding constant (Kd=89 nM) measured by isothermal titration calorimetry. PFI-3 does not phenocopy the growth inhibitory effects of SMARCA2 knockdown in lung cancer[1]. Exposure of embryonic stem cells to PFI-3 leads to deprivation of stemness and deregulates lineage specification. Furthermore, differentiation of trophoblast stem cells in the presence of PFI-3 is markedly enhanced[2]. PFI-3 binds to certain family VIII bromodomains while displaying significant, broader bromodomain family selectivity. The high specificity of PFI-3 for family VIII is achieved through a novel bromodomain binding mode of a phenolic headgroup that leads to the unusual displacement of water molecules that are generally retained by most other bromodomain inhibitors reported to date[3]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.