| Cas No.: | 2138300-40-8 |
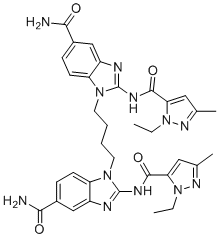
| Chemical Name: | 1,1'-(butane-1,4-diyl)bis(2-(1-ethyl-3-methyl-1H-pyrazole-5-carboxamido)-1H-benzo[d]imidazole-5-carboxamide) |
| SMILES: | C(N1C2=CC=C(C(N)=O)C=C2N=C1NC(C1N(CC)N=C(C)C=1)=O)CCCN1C2=CC=C(C(N)=O)C=C2N=C1NC(C1N(CC)N=C(C)C=1)=O |
| Formula: | C34H38N12O4 |
| M.Wt: | 678.758 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | 1. Ramanjulu JM, et al. Nature. 2018 Nov 7. doi: 10.1038/s41586-018-0705-y. |
| Description: | STING agonist-4 is an stimulator of Interferon Genes (STING) receptor agonist with an apparent inhibitory constant (IC50) of 20 nM. STING agonist-4 is a two symmetry-related amidobenzimidazole (ABZI)-based compound to create linked ABZIs (diABZIs) with enhanced binding to STING and cellular function[1]. |
| Target: | IC50: 20 nM (STING agonist-4)[1] |
| In Vitro: | STING agonist-4 (Compound 2) (0.3-30 μM; 2 hours) causes phosphorylation of IRF3 and STING that is inhibited by the TBK1 inhibitor BX795 and induces dose-dependent secretion of IFN-β with an EC50 of 3.1 μM[1]. STING agonist-4 (Compound 2) (0.001 nM-1 μM) inhibits binding of full-length STING to the solid support with an apparent dissociation constant (Kd) of approximately 1.6 nM[1]. STING agonist-4 (Compound 2) (0-100 μM) is 18-fold more potent than cGAMP (an endogenous STING ligand), with an EC50 of 53.9 μM[1]. STING agonist-4 (Compound 2) (3 μM; 4 hours) promotes production of interferon γ-induced protein 10 (IP-10), IL-6 and TNF-α by a mechanism that is dependent on STING-mediated activation of TBK1[1]. Cell Viability Assay[1] Cell Line: Human peripheral blood mononuclear cells (PBMCs) Concentration: 0.3 μM, 1 μM, 3 μM, 10 μM and 30 μM Incubation Time: 2 hours Result: Caused phosphorylation of IRF3 and STING and induced secretion of IFN-β. |
| References: | [1]. Ramanjulu JM et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018 Dec;564(7736):439-443. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.