| Cas No.: | 1186372-20-2 |
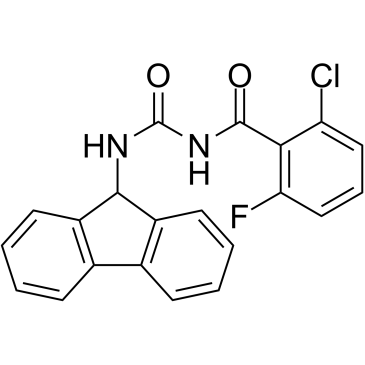
| Chemical Name: | 2-Chloro-N-[(9H-fluoren-9-ylamino)carbonyl]-6-fluorobenzamide |
| Synonyms: | TMN355 ;TMN-355 ;TMN 355 |
| SMILES: | O=C(NC(=O)C1=C(Cl)C=CC=C1F)NC2C3C(=CC=CC=3)C4=C2C=CC=C4 |
| Formula: | C21H14N2O2FCl |
| M.Wt: | 380.79946 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | [1]. Ramachandran S, et al. Cyclophilin A enhances macrophage differentiation and lipid uptake in high glucose conditions: a cellular mechanism for accelerated macro vascular disease in diabetes mellitus. Cardiovasc Diabetol. 2016 Nov 3;15(1):152. |
| Description: | TMN355 is a potent chemical cyclophilin A inhibitor and reduces foam cell formation and cytokine secretion. TMN355 is used for atherosclerosis[1]. |
| Target: | cyclophilin A[1] |
| In Vitro: | TMN 355 (0.5-10 μM; 3-9 hours) results in 75.9% reduction of cyclophilin A protein expression. And 1 μM TMN 355 inhibits cyclophilin A after 6 h of activation[1]. Western Blot Analysis[1] Cell Line: THP cell lines Concentration: 0.5, 1, 2.5, 5 and 10 μM Incubation Time: 3, 6 and 9 hours Result: Resulted in 75.9% reduction of cyclophilin A protein expression. |
| References: | [1]. Ramachandran S, et al. Cyclophilin A enhances macrophage differentiation and lipid uptake in high glucose conditions: a cellular mechanism for accelerated macro vascular disease in diabetes mellitus. Cardiovasc Diabetol. 2016 Nov 3;15(1):152. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.