| Cas No.: | 1884461-72-6 |
| Chemical Name: | Ensifentrine |
| Synonyms: | Ensifentrine;RPL 554;3E3D8T1GIX;RPL554;Ensifentrine [INN];Ensifentrine [USAN];Ensifentrine (USAN/INN);BCP31840;WHO 10726;SB19810;D11743;Q7277486;9,10-Dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carba |
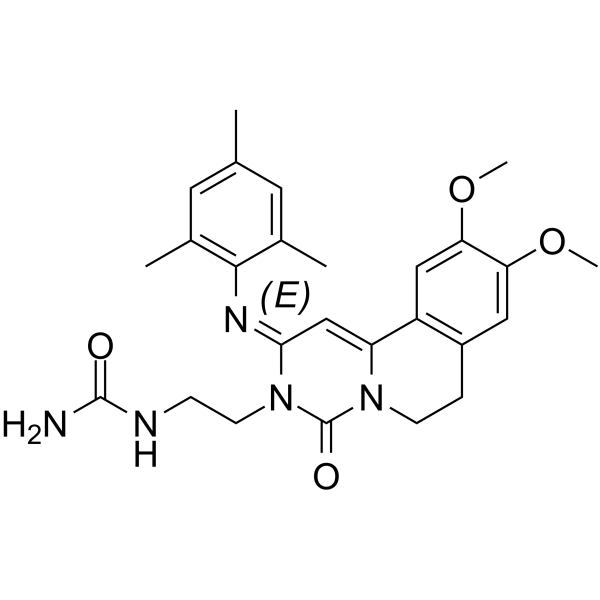
| SMILES: | O=C1N(C([H])([H])C([H])([H])N([H])C(N([H])[H])=O)/C(/C([H])=C2C3=C([H])C(=C(C([H])=C3C([H])([H])C([H])([H])N21)OC([H])([H])[H])OC([H])([H])[H])=N/C1C(C([H])([H])[H])=C([H])C(C([H])([H])[H])=C([H])C=1C([H])([H])[H] |
| Formula: | C26H31N5O4 |
| M.Wt: | 477.5554 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | [1]. Victoria Boswell-Smith, et al. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]. J Pharmacol Exp Ther. 2006 Aug;318(2):840-8. [2]. Henrik Watz, et al. Symptom Improvement Following Treatment with the Inhaled Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine in Patients with Moderate to Severe COPD - A Detailed Analysis. Int J Chron Obstruct Pulmon Dis. 2020 Sep 16;15:2199-2206. |
| Description: | Ensifentrine (RPL-554) is an inhaled first-in-class dual inhibitor of phosphodiesterase 3 (PDE3) and PDE4 with IC50s of 0.4 nM and 1479 nM, respectively. Ensifentrine has bronchoprotective and anti-inflammatory activities. Ensifentrine can be used for chronic obstructive pulmonary disease (COPD) research[1][2]. |
| Target: | PDE3:0.4 nM (IC50) PDE4:1479 nM (IC50) |
| In Vivo: | Ensifentrine (RPL-554; 10 mg/kg; Oral administration; once) significantly inhibits eosinophil recruitment following antigen challenge in ovalbumin-sensitized guinea pigs[1]. The inhalation of dry powder containing Ensifentrine by conscious guinea pigs (25% in micronized lactose) 1.5 h before antigen exposure significantly inhibits the recruitment of eosinophils to the airways[1]. Exposure of conscious guinea pigs to inhalation of dry powder containing Ensifentrine (2.5%) in micronized lactose significantly inhibits histamine-induced plasma protein extravasation in the trachea and histamine-induced bronchoconstriction over a 5.5-h period[1]. Animal Model: Male Dunkin Hartley guinea pigs (200-300 g) injected with ovalbumin[1]. Dosage: 10 mg/kg Administration: Oral administration; once Result: Significantly inhibits eosinophil recruitment following antigen challenge in ovalbumin-sensitized guinea pigs. |
| In Vitro: | Ensifentrine (RPL-554) inhibits, in a concentration-dependent manner, lipopolysaccharide-induced TNF-α release from human monocytes (IC50 of 0.52 μM) and proliferation of human mononuclear cells to phytohemagglutinin (IC50 of 0.46 μM)[1]. Electrical field stimulation-induced contraction of guinea pig superfused isolated tracheal preparations is significantly inhibited by Ensifentrine (10 μM). Contractile responses are suppressed for up to 12 h after termination of superfusion with RPL-554 demonstrating a long duration of action[1]. |
| References: | [1]. Victoria Boswell-Smith, et al. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]. J Pharmacol Exp Ther. 2006 Aug;318(2):840-8. [2]. Henrik Watz, et al. Symptom Improvement Following Treatment with the Inhaled Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine in Patients with Moderate to Severe COPD - A Detailed Analysis. Int J Chron Obstruct Pulmon Dis. 2020 Sep 16;15:2199-2206. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.