| Cas No.: | 2326521-71-3 |
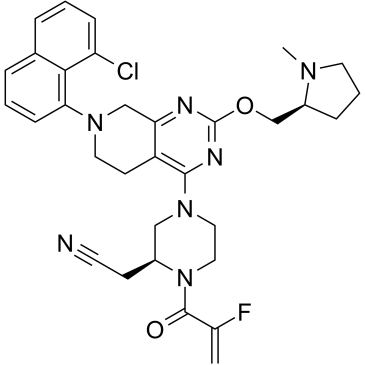
| Chemical Name: | 2-((S)-4-(7-(8-chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile |
| Synonyms: | MRTX849,MRTX-849,MRTX 849 |
| SMILES: | N#CC[C@@H]1N(C(C(F)=C)=O)CCN(C2=C3C(CN(C4=C5C(Cl)=CC=CC5=CC=C4)CC3)=NC(OC[C@H]6N(C)CCC6)=N2)C1 |
| Formula: | C32H35ClFN7O2 |
| M.Wt: | 604.12 |
| Sotrage: | 2 years -20°C Powder, 2 weeks4°C in DMSO,6 months-80°C in DMSO |
| Publication: | [1]. Christensen JG, et al. The KRASG12C Inhibitor, MRTX849, Provides Insight Toward Therapeutic Susceptibility of KRAS Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2019 Oct 28. pii: CD-19-1167. [2]. Kyriakos P. Papadopoulos, et al. A ph |
| Description: | MRTX849 is a potent, orally-available, and mutation-selective covalent inhibitor of KRAS G12C with potential antineoplastic activity. MRTX849 covalently binds to KRAS G12C at the cysteine at residue 12, locks the protein in its inactive GDP-bound conformation, and inhibits KRAS-dependent signal transduction[1][2]. |
| In Vivo: | MRTX849 inhibits KRASG12C signaling in cell lines harboring this mutation, and results in tumor regression in a broad spectrum of KRASG12C animal models[2]. |
| In Vitro: | In cell lines, MRTX849 inhibits growth and viability of cells harboring KRASG12C mutations, but not in cells with other mutant forms or of wild-type KRAS[2]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.