| Cas No.: | 1374640-70-6 |
| Chemical Name: | Rociletinib |
| Synonyms: | CO 1686;CO-1686 (AVL-301);CO-1686;N-(3-((2-((4-(4-Acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)acrylamide;N-[3-[[2-[4-(4-acetylpiperazin-1-yl)-2-methoxyanilino]-5-(trifluoromethyl)pyrimidin-4-yl]amino]phenyl]prop-2-enamide;Pyridostadin;Rociletinib;Rociletinib(CO-1686);(N-(3-(2-(4-(4-acetylpiperazin-1-yl)-2-methoxyphenylamino)-5-(trifluoromethyl)pyrimidin-4-ylamino)phenyl)acrylamide);AVL-301;CNX-419;N-(3-((2-((4-(4-Acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)prop-2-enamide;S7284,CNX-419,AVL-301;CO-1686(Rociletinib);CO1686;72AH61702G;Rociletinib [USAN:INN];Tube721;Rociletinib (USAN/INN);GTPL7966;SC |
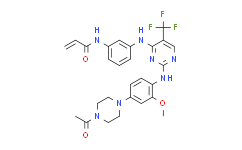
| SMILES: | FC(C1=C([H])N=C(N=C1N([H])C1C([H])=C([H])C([H])=C(C=1[H])N([H])C(C([H])=C([H])[H])=O)N([H])C1C([H])=C([H])C(=C([H])C=1OC([H])([H])[H])N1C([H])([H])C([H])([H])N(C(C([H])([H])[H])=O)C([H])([H])C1([H])[H])(F)F |
| Formula: | C27H28F3N7O3 |
| M.Wt: | 555.55 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Rociletinib (CO-1686) is an orally delivered kinase inhibitor that specifically targets the mutant forms of EGFR including T790M, and the Ki values for EGFRL858R/T790M and EGFRWT are 21.5 nM and 303.3 nM, respectively. |
| In Vivo: | Rociletinib (CO-1686) (100 mg/kg/day, p.o.) demonstrates anti-tumor activity in NSCLC EGFR mutant xenograft models. Rociletinib (CO-1686) (50 mg/kg bid, p.o.) demonstrates anti-tumor activity in human EGFR-L858R and EGFR-L858R-T790M expressing transgenic mice[1]. |
| In Vitro: | Rociletinib (CO-1686) (0.1 μM) inhibits EGFR potently and irreversibly, and inhibits more than 50% of 23 targets. Rociletinib potently and selectively inhibits growth of NSCLC cells expressing mutant EGFR and induces apoptosis. Rociletinib resistant NSCLC cell lines are sensitive to AKT inhibition[1]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.