| Cas No.: | 1857340-78-3 |
| Chemical Name: | 4A3-SC8 |
| Synonyms: | 4A3 SC8,4A3SC8 |
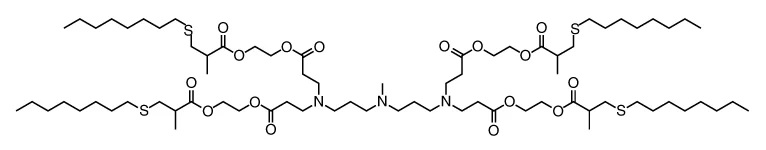
| SMILES: | O=C(OCCOC(=O)C(C)CSCCCCCCCC)CCN(CCC(=O)OCCOC(=O)C(C)CSCCCCCCCC)CCCN(C)CCCN(CCC(=O)OCCOC(=O)C(C)CSCCCCCCCC)C CC(=O)OCCOC(=O)C(C)CSCCCCCCCC |
| Formula: | C75H139N3O16S4 |
| M.Wt: | 1467.2 |
| Purity: | >95% |
| Sotrage: | -20 |
| Publication: | 1 Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo-Journal of Controlled Release by Hu Xionga, Shuai Liua, Tuo Weia, Qiang Chenga, Daniel J. Siegwarta||||| 2 Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery--Nature Protocols volume 18, pages265–291 (2023) |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.