| Cas No.: | 159432-28-7 |
| Chemical Name: | 1-7-Angiotensin II,5-L-isoleucine-7-D-alanine- |
| Synonyms: | (D-Ala7)-Angiotensin I/II (1-7) A-779;(D-ALA7)-ANGIOTENSIN I/II (1-7);1-7-Angiotensin II,5-L-isoleucine-7-D-alanine-;(D-Ala?)-AngiotensinI;A-779;II (1-7) A-779;II (1-7);H-ASP-ARG-VAL-TYR-ILE-HIS-D-ALA-OH;DRVYIHA;DRVYIH{d-ALA};ASP-ARG-VAL-TYR-ILE-HIS-D-ALA;(D-ALA7)-ANGIOTENSIN I |
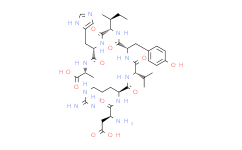
| SMILES: | CC[C@H](C)[C@H](NC([C@H](CC1=CC=C(O)C=C1)NC([C@@H](NC([C@H](CCCNC(N)=N)NC([C@@H](N)CC(O)=O)=O)=O)C(C)C)=O)=O)C(N[C@@H](CC1=CN=CN1)C(N[C@H](C)C(O)=O)=O)=O |
| Formula: | C39H60N12O11 |
| M.Wt: | 872.96750831604 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | A 779 is a specific antagonist of G-protein coupled receptor (Mas receptor), which is an Ang1-7 receptor distinct from the classical AngII. |
| In Vivo: | Infusion of Ang(1-7) and A-779 (400 ng/kg/min, s.c.) alone or combined for 6 weeks does not prevent uterus atrophy or inhibit the body weight gain of OVX rats. A-779 markedly elevates serum bone specific alkaline phosphatase (BALP), telopeptides of collagen type I (CTX), tartarate resistant acid phosphatase (TRAcP 5b), osteocalcin (OC) and urinary deoxypyridinoline (DPD). Infusion of Ang(1-7) and/or A-779 does not significantly change serum minerals concentrations in sham or OVX groups. A-779 in the OVX animals does not change AngII, Ang(1-7), AT1R, AT2R, ACE, ACE-2, Mas receptor, RANKL and OPG proteins expressions in relation to OVX group, while AngII (P < 0.05), AT1R (P < 0.05), ACE (P < 0.01) and RANKL (P < 0.01) expressions are significantly higher and Ang(1-7), AT2R, ACE-2, MasR and OPG are significantly (P < 0.01) lower than sham group. Blocking of the G-protein coupled receptor (Mas) by A-779 markedly abolishes Ang(1-7) favorable effects on bone health suggesting the vital role of Mas receptor in mediating Ang(1-7) osteo-protective effects[1]. Inhibition of Ang1-7 cascade by A-779 (400 ng/kg/min) significantly eradicates captopril protective effects on bone metabolism, mineralization and micro-structure. A-779 also restores OVX effects on RANKL expression and ACE-1/AngII/AT1R cascade and down-regulates OPG expression and ACE-2/Ang1-7/Mas pathway[2]. |
| In Vitro: | A-779 inhibits the effect of Ang-(1-7), which suppresses the proliferating cell nuclear antigen (PCNA) protein expression up-regulated by Ang II, but A-779 alone has no effect to induce proliferation and migration of VSMCs. Pretreatment with Ang-(1-7) significantly retards Ang II-induced inflammatory responses of VSMCs associated with up-regulated MCP-1, VCAM-1 and IL-1β expressions, and this effect of Ang-(1-7) is blocked by A-779. But A-779 alone has no effect to induce inflammatory response of VSMCs. Pretreatment VSMCs with Ang-(1-7) for 5 min significantly inhibits Akt and ERK1/2 phosphorylation induced by Ang II, and this effect is also blocked by A-779, but alone has no effect to induce phosphorylation of Akt and ERK1/2 in VSMCs[3]. |
| Animal Administration: | A bilateral OVX operation is use to induce postmenopausal osteoporosis in female Wistar albino rats. The animals are generally anesthetized using single IP injection of ketamine + xylazine combination (80 mg/kg and 5 mg/kg; respectively). After ligation, the ovaries are excised from a longitudinal incision made on the dorsolateral body region. Sham rats have the same procedure without the ligation and excision. The risk of postoperative infection is eliminated by applying fusidic acid topical antibiotic cream twice weekly for 4 weeks. Following the sham and OVX operations, animals are divided into eight groups (n = 8/group) including, Sham, Sham + Ang(1-7), Sham + A-779, Sham + Ang(1-7)+A-779, OVX, OVX + Ang(1-7), OVX + A-779 and OVX + Ang(1-7)+A-779. Eight weeks following sham and OVX operations, osmotic pumps are implanted subcutaneously to deliver Ang(1-7) (200 ng/kg/min), A-779 (400 ng/kg/min) or a mixture of Ang(1-7) and A-779 (200 ng/kg/min and 400 ng/kg/min; respectively) for 6 consecutive weeks. Body weights of all animals are monitored and recorded at the beginning and every week throughout the study. Animals' general health is maintained during periods of treatments. At the end of treatments periods, animals are placed fasting in metabolic cages for 16 hr and urine samples are collected and frozen at −70°C. Then, the blood samples are collected by cardiac puncture under ketamine + xylazine anesthesia and serum samples are obtain by centrifugation at 4000 RPM to. Animals are then sacrificed by decapitation and the uterine tissues and femoral bones are removed and cleaned. Uterine tissues are cleaned from fats and the wet weights are determined directly and expressed as g/100 g body weight. In each rat, both femurs are removed and cleaned from soft tissues. The left femoral bone samples are frozen at −70°C until analyzed, while the right femoral bones are kept in 10% formalin for mico-CT and minerals analysis. |
| References: | [1]. Abuohashish HM, et al. Angiotensin (1-7) ameliorates the structural and biochemical alterations of ovariectomy-induced osteoporosis in rats via activation of ACE-2/Mas receptor axis. Sci Rep. 2017 May 23;7(1):2293. [2]. Abuohashish HM, et al. ACE-2/Ang1-7/Mas cascade mediates ACE inhibitor, captopril, protective effects in estrogen-deficient osteoporotic rats. Biomed Pharmacother. 2017 Aug;92:58-68. [3]. Yan WF, et al. Effects and related mechanism of angiotensin-(1-7) on Toll-like receptor 4-mediated oxidative stress in human umbilical vein endothelial cells. Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Mar 24;45(3):223-229. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.