| Cas No.: | 956104-40-8 |
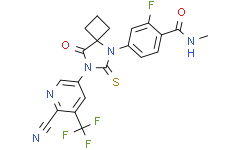
| Chemical Name: | Apalutamide (ARN-509) |
| Synonyms: | ARN-509;ARN 509;4-[7-[6-cyano-5-(trifluoromethyl)pyridin-3-yl]-8-oxo-6-sulfanylidene-5,7-diazaspiro[3.4]octan-5-yl]-2-fluoro-N-methylbenzamide;Apalutamide;AR509;4-(7-(6-Cyano-5-(trifluoromethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspiro(3.4)octan-5-yl)-2-fluoro-N-methylbenzamide;4-[7-(6-cyano-5-trifluoromethylpyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-2-fluoro-N-methylbenzamide;4-[7-[6-cyano-5-(trifluoromethyl)pyridin-3-yl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]octa;Benzamide,4-[7-[6-cyano-5-(trifluoromethyl)-3-pyridinyl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-2-fluoro-N-methyl;4-[7-[6-Cyano-5-(trifluoromethyl)pyridin-3-yl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]octan-5-yl]-2-fluoro-N-methylbenzamide;ARN509;4T36H88UA7;4-(7-(6-CYANO-5-(TRIFLUOROMETHYL)PYRIDIN-3-YL)-8-OXO-6-THIOXO-5,7-DIAZASPIRO[3.4]OCTAN-5-YL)-2-FLUORO-N-METHYLBENZAMIDE;Erleada;4-(7-(6-cyano-5-(trifluoroMethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspirooctan-5-yl)-2-fluoro-N-MethylbenzaMide;Apalutamide [INN];4-{7-[6-cyano-5-(tr |
| SMILES: | S=C1N(C2C([H])=NC(C#N)=C(C(F)(F)F)C=2[H])C(C2(C([H])([H])C([H])([H])C2([H])[H])N1C1C([H])=C([H])C(C(N([H])C([H])([H])[H])=O)=C(C=1[H])F)=O |
| Formula: | C21H15F4N5O2S |
| M.Wt: | 477.43 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Apalutamide (ARN-509) is a potent and competitive androgen receptor (AR) antagonist, binding AR with an IC50 of 16 nM. |
| Target: | IC50: 16 nM (Androgen receptor)[1] |
| In Vivo: | Apalutamide (ARN-509) exhibits low systemic clearance, high oral bioavailability and long plasma half-life in both mouse and dog, supporting once-daily oral dosing. Consistent with its long terminal-half-life, Apalutamide steady-state plasma-levels increases in repeat-dose studies, resulting in high C24hr levels and low peak:trough ratios (ratio:2.5). Castrate male mice bearing LNCaP/AR xenograft tumors are treated with either Apalutamide at doses of 1, 10 or 30 mg/kg/day. Thirteen of 20 Apalutamide (30 mg/kg/day)-treated animals exhibit >50% reduction in tumor-volume at day 28 versus 3 of 19 MDV3100 (30 mg/kg/day)-treated mice[1]. |
| In Vitro: | Apalutamide (ARN-509) also exhibits low micromolar affinity (IC50 3 μM) for the GABAA receptor in radioligand binding-assays and thus may potentially antagonize GABAA at therapeutic dose levels[1]. Apalutamide is a potent androgen receptor (AR) antagonist that targets the AR ligand-binding domain and prevents AR nuclear translocation, DNA binding, and transcription of AR gene targets[2]. |
| Cell Assay: | Trypsinized VCaP cells are adjusted to a concentration of 100,000 cells per mL in phenol-red-free RPMI 1640 (with 5% CSS), and dispensed in 16 µL aliquots into CellBIND 384 well plates. Cells are incubated for 48 hours, after which ligand is added in a 16 µL volume to the RPMI culture medium. For the antagonist mode assay, the ligands are diluted in culture medium also containing 30 pM R1881. After 7 days’ incubation, 16 µL of CellTiter-Glo Luminescent Cell Viability Assay is added and Relative Luminescence Units (RLUs) measured[1]. |
| Animal Administration: | Mice[1] In vivo xenograft experiments to determine anti-tumor response are carried out in SHO SCID male mice. Mice are orchiectomized under isoflorane anesthesia and are given 2-3 days to recover prior to tumor cell injection. LNCaP/AR(cs) cells are suspended in 50% RPMI, 50% Matrigel, and 5×106 cells/xenograft are injected in a volume of 100 μL. Animals are observed weekly until tumor growth is apparent. From 24 d post-injection, tumors are measured weekly, and after 40-60 days post-injection, animals are randomized into cohorts of equivalent mean (150-250 mm3) and range tumor burden. All compounds (e.g., Apalutamide, 30 mg/kg per day) are administered daily by oral gavage. Statistical analyses are performed using Graphpad Prism. |
| References: | [1]. Clegg NJ, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012 Mar 15;72(6):1494-503. [2]. Smith MR, et al. Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort. Eur Urol. 2016 May 6. pii: S0302-2838(16)30133 |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.