| Cas No.: | 1370554-01-0 |
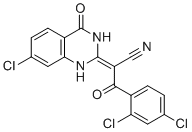
| Chemical Name: | 2-(7-chloro-4-oxo-3,4-dihydroquinazolin-2(1H)-ylidene)-3-(2,4-dichlorophenyl)-3-oxopropanenitrile |
| SMILES: | N#C/C(=C1/NC2C=C(Cl)C=CC=2C(=O)N/1)/C(=O)C1C(Cl)=CC(Cl)=CC=1 |
| Formula: | C17H8Cl3N3O2 |
| M.Wt: | 392.623 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Ciliobrevin D is a cell-permeable, reversible and specific inhibitor of AAA+ ATPase motor cytoplasmic dynein. Ciliobrevin D inhibits Hedgehog (Hh) signaling and primary cilia formation. Ciliobrevin D inhibits dynein-dependent microtubule gliding and ATPase activity in vitro[1][2]. |
| Target: | Cytoplasmic dynein[1][2] |
| In Vivo: | Knockdown of Dync1h1 or inactivation of dynein 1 by Ciliobrevin D in the testis in vivo perturbs spermatogenesis. Knockdown of Dync1h1 or the use of Ciliobrevin D to inactivate dynein 1 in the testis in vivo perturbs MT organization through changes in the spatial expression of EB1, perturbs F-actin organization, and perturbs distribution of adhesion protein complexes at the BTB, leading to a loss of BTB integrity. F-actin disorganization in the seminiferous epithelium following Dync1h1 knockdown or dynein 1 inactivation by Ciliobrevin D is mediated by changes in the spatiotemporal expression of actin regulatory proteins Arp3 and Eps8[3]. |
| In Vitro: | Cells treated with Ciliobrevin D exhibits abnormal (unfocused, multipolar, or collapsed) spindles with disrupted γ-tubulin localization in NIH-3T3 cells. Similar Ciliobrevin-induced spindle defects are observed in HeLa cells, although to a lesser extent. Ciliobrevin D addition also reversibly disrupts the pre-formed spindles of metaphase-arrested cells and reduces overall microtubule levels[1]. .Ciliobrevin D reversibly inhibits melanosome aggregation, but the non-cilia-disrupting derivative had no discernible effect at comparable doses. Ciliobrevin D similarly abrogates the movement of peroxisomes in Drosophila S2 cells[1]. |
| References: | [1]. Firestone AJ, et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012 Mar 18;484(7392):125-9. [2]. Miao Y, et al. Dynein promotes porcine oocyte meiotic progression by maintaining cytoskeletal structures and cortical granule arrangement. Cell Cycle. 2017;16(21):2139-2145. [3]. Wen Q, et al. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am J Physiol Endocrinol Metab. 2018 Nov 1;315(5):E924-E948. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.