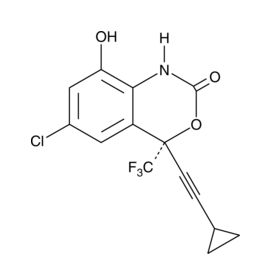
| Cas No.: | 205754-33-2 |
| Chemical Name: | (4S)-6-Chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-8-hydroxy-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one; 8-Hydroxyefavirenz |
| SMILES: | ClC1=CC(O)=C2C([C@@](C#CC3CC3)(C(F)(F)F)OC(N2)=O)=C1 |
| Formula: | C14H9ClF3NO3 |
| M.Wt: | 331.67 |
| Purity: | >97% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | 8-hydroxy Efavirenz is a major oxidative metabolite of the non-nucleoside reverse transcriptase inhibitor efavirenz.8-hydroxy Efavirenz is formed when efavirenz is metabolized by the cytochrome P450 (CYP) isoform CYP2B6. It induces apoptosis in rat primary hippocampal neurons and loss of dendritic spines in rat primary hippocampal neuronal cultures when used at a concentration of 0.01 μM.In active antiretroviral therapy antiretroviral drugs are employed for the restoration of a functional immune system in patients suffering from the acquired immunodeficiency syndrome. However, potential adverse effects of such compounds to brain cells are discussed in connection with the development of neurocognitive impairments in patients. To investigate potential effects of antiretroviral drugs on cell viability and the glycolytic flux of brain cells, astrocyte-rich primary cultures were exposed to various antiretroviral compounds, including the non-nucleoside reverse transcriptase inhibitor efavirenz. In a concentration of 10 μM, neither efavirenz nor any of the other investigated antiretroviral compounds acutely compromised the cell viability nor altered glucose consumption or lactate production. In contrast, the primary metabolite of efavirenz, 8-hydroxy-efavirenz, stimulated the glycolytic flux in viable astrocytes in a time- and concentration-dependent manner with half-maximal and maximal effects at concentrations of 5 and 10 μM, respectively. The stimulation of glycolytic flux by 8-hydroxy-efavirenz was not additive to that obtained for astrocytes that were treated with the respiratory chain inhibitor rotenone and was abolished by removal of extracellular 8-hydroxy-efavirenz. In a concentration of 10 μM, 8-hydroxy-efavirenz and efavirenz did not affect mitochondrial respiration, while both compounds lowered in a concentration of 60 μM significantly the oxygen consumption by mitochondria that had been isolated form cultured astrocytes, suggesting that the stimulation of glycolytic flux by 8-hydroxy-efavrienz is not caused by direct inhibition of respiration. The observed alteration of astrocytic glucose metabolism by 8-hydroxy-efavirenz could contribute to the adverse neurological side effects reported for patients that are chronically treated with efavirenz-containing medications. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.