| Cas No.: | 126150-97-8 |
| Chemical Name: | Bapta-AM |
| Synonyms: | Tetrakis(acetoxymethyl) 2,2',2'',2'''-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(azanetriyl))tetraacetate;BAPTA-AM;1,2-BIS (2-AMINOPHENOXY)ETHANE-N,N,N',N'-TETRAACETIC ACID, TETRAACETOXYMETHYL ESTER;acetyloxymethyl 2-[N-[2-(acetyloxymethoxy)-2-oxoethyl]-2-[2-[2-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]phenoxy]ethoxy]anilino]acetate;BAPTA AM;BAPTA-Acetoxymethyl ester;DAPTA-AM;1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic Acid Tetrakis(acetoxymethyl) Ester;O,O'-Bis(2-aminophenyl)ethyleneglycol-N,N,N',N'-te traacetic Acid, Tetraacetoxymethyl Ester 50mM;tetraacetoxymethyl ester;1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester);1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester);1,2-BIS(2-AMINOPHENOXY)ETHANE-N,N,N,N-TETRAACETIC ACID ACETOXYMETHYL ESTER;Tetrakis(acetoxyMethyl) 1,2-Bis(2-aMinophenoxy)ethane-N,N,N',N'-tetraacetate;1,2-BIS(O-AMINOPHENOXY)ETHANE-N,N,N',N'-TETRAACETIC ACID TETRA(ACETOXYMETHYL) ESTER;1,2-BIS(2-AMINOPHENOXY)ETHANE-N,N,N,N-TETRAACETIC ACID ACETOXYMETHYL ESTER(BAPTA-AM);1,2-BIS(2-AMINOPHENOXY)ETHANE-N,N,N',N'-TETRAACETIC ACID TETRAKIS(ACETOXY-METHYL) ESTER;BAPTA AM, >=98%;BAPTA, AM *CAS 126150-97-8*;BAPTA, AM *UltraPure Grade* *CAS 126150-97-8*;BAPTA/AM;Tetrakis(acetoxymethyl) 1,2-Bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetate;(acetyloxy)methyl 2-({2-[(acetyloxy)methoxy]- |
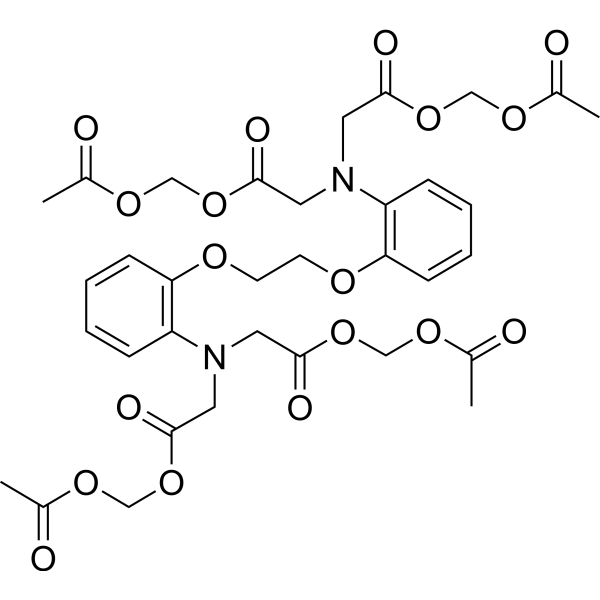
| SMILES: | O(C([H])([H])C([H])([H])OC1=C([H])C([H])=C([H])C([H])=C1N(C([H])([H])C(=O)OC([H])([H])OC(C([H])([H])[H])=O)C([H])([H])C(=O)OC([H])([H])OC(C([H])([H])[H])=O)C1=C([H])C([H])=C([H])C([H])=C1N(C([H])([H])C(=O)OC([H])([H])OC(C([H])([H])[H])=O)C([H])([H])C(=O)OC([H])([H])OC(C([H])([H])[H])=O |
| Formula: | C34H40N2O18 |
| M.Wt: | 764.6840 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | BAPTA-AM is an intracellular calcium chelator. |
| In Vitro: | The permeable calcium chelator BAPTA/AM is known to prevent free radical-mediated toxicity promote apoptosis in non-neuronal cells and produce a beneficial effect in neuronal cells by protecting neurons from ischemic damage. In addition, it has been suggested that BAPTA/AM induces a late, but not early, increase of intracellular calcium in I-IL-60 neoplastic cells. Mixed cortical cell cultures (DIV 13-16) exposed to 10μM BAPTA/AM for 24- or 48-hr show moderate (45-70%) neuronal injury as evaluated by increased LDH release into the bathing medium after 24-48-hr. Exposure of cortical cultures to 3-10 μM BAPTA/AM for 48-hr evoke dose-dependent neuronal damage[1]. |
| Cell Assay: | Neuronal injury is quantitatively estimated by measuring lactate dehydrogenase (LDH) released from damaged cells into the bathing medium 24- or 48-hr after the 10 μM BAPTA/AM treatment. The morphological findings are confirmed by staining with neuron-specific enolase (NSE) antibody and tryphan blue[1]. |
| References: | [1]. Wie MB, et al. BAPTA/AM, an intracellular calcium chelator, induces delayed necrosis by lipoxygenase-mediated free radicals in mouse cortical cultures. Prog Neuropsychopharmacol Biol Psychiatry. 2001 Nov;25(8):1641-59. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.