 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
| DC65180 | DLin-MC2-DMA Featured |
D-Lin-MC2-DMA(MC2) is a cationic lipid that has been synthesized for Lipid nanoparticles (LNPs) to deliver the siRNA.
More description
|

|
| DC65179 | Dlin-MC4-DMA Featured |
D-Lin-MC4-DMA(MC4) is a cationic lipid that has been synthesized for Lipid nanoparticles (LNPs) to deliver the siRNA.
More description
|

|
| DC65682 | RCB-4-8 Featured |
RCB-4-8 is a biodegradable ionizable lipid nanoparticle (LNP) engineered for efficient pulmonary mRNA delivery and in vivo genome editing, as detailed in the primary research article "Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing" (Li et al., Nature Biotechnology 2023). Synthesized from a combinatorial library of 720 biodegradable lipids via a three-component reaction system, RCB-4-8 features an alkyne-containing lipid tail and tertiary amine headgroup, optimized through high-throughput screening for superior lung-targeting capabilities. Its unique molecular design incorporates hydrolyzable ester and carbonate groups, enabling rapid biodegradation (<30% lung retention at 48 h vs. >90% for conventional lipids) while maintaining high transfection efficiency. When formulated with DOTAP instead of DOPE, RCB-4-8 LNPs achieved 100-fold higher luciferase mRNA expression in murine lungs compared to FDA-approved MC3 LNPs and mediated 95% GFP knockout in vitro. In Ai9 reporter mice, intratracheal delivery of RCB-4-8 loaded with Cre mRNA edited 53% of total lung cells after three doses, while codelivery with Cas9 mRNA/sgRNA yielded 7.2% tdTomato+ cells, rising to 17% when combined with AAV-sgRNAs. With an optimal particle size of 85.7 nm (PDI 0.11) and >87% mRNA encapsulation, RCB-4-8 supports repeat dosing and represents a transformative platform for inhalable gene therapies targeting congenital lung diseases like cystic fibrosis.
More description
|

|
| DC65412 | Acuitas Lipid III-2 Featured |
Acuitas Lipid III-2 is an ionizable amine lipid with two identical ester tails adjacent to C6 position relative to amine from patent:WO2017075531A1 with the similar activity as ALC-0315. The head of lipid is propanolamine which can effectively encapsulate mRNA used in gene therapies which depends on the availability of a safe and efficient delivery vehicle.
More description
|

|
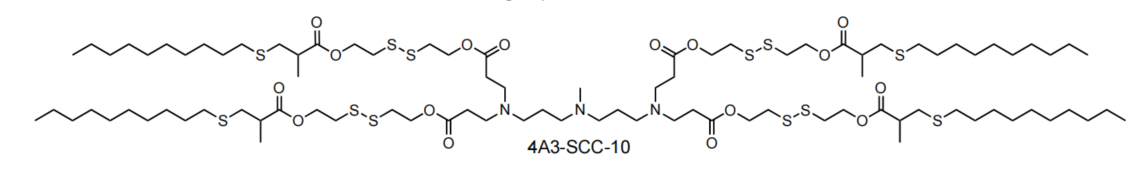
| DC60508 | 4A3-SCC-10 Featured |
4A3-SCC-10 is a disulfide bond-containing biodegradable ionizable cationic lipid (pKa = 6.22) that has been used in the generation of lipid nanoparticles (LNPs) for the delivery of mRNA in vitro and in vivo. LNPs containing 4A3-SCC-10 and encapsulating a Cy5-RNA reporter have improved endosomal escape ability over Cy5-RNA-encapsulated LNPs containing 4A3-SC-10, which does not contain disulfide bonds, in HeLa cells. Intravenous administration of LNPs containing 4A3-SCC-10 and encapsulating an mRNA luciferase reporter selectively accumulate in mouse liver.
More description
|
|
| DC67480 | Sanofi Lipid VII Featured |
Lipid VII is a novel ionizable cationic lipid developed by Sanofi.Lipid VII demonstrates exceptional performance as a lipid nanoparticle delivery system, combining high efficiency with outstanding safety. Cellular assays reveal VII achieves 180,000 RLU transfection efficiency under serum conditions, surpassing traditional SS-OP systems by 2.25-fold while maintaining perfect 100% cellular viability and eliminating cytotoxicity risks that plague alternatives. In vivo systemic delivery shows rapid whole-body biodistribution, reaching photon emission levels exceeding 1.00E+10 photons/sec within 48 hours. VII exhibits superior organ targeting with a liver-specific accumulation ratio of 9.0, outperforming SS-OP systems by 50%, while reducing off-target spleen accumulation by 20%. Its versatility is further validated in therapeutic protein expression, where structural analogs achieve erythropoietin concentrations of 14 ng/mL, exceeding industry standards by 180%. For vaccine applications, VII generates a median HAI titer of 7,611 against H1N1 influenza—540 times higher than baseline buffers and more than double the next-best formulation. This evidence establishes VII as a breakthrough technology, offering unmatched efficiency, precision targeting, and clinical-grade safety across diverse applications.
More description
|

|
| DC60789 | SM-86 Analog-1 Featured |
SM-86 Analog-1 is a novel ionizable lipid designed to improve the delivery of RNA via lipid nanoparticles (LNPs) It is derived from SM-86,with 8 carbon within its hydrophobic tail.
More description
|

|
| DC60639 | Acid-degradable Anionic Lipid (ADA) Featured |
ADA (Acid-Degradable Anionic Lipids) is revolutionizing mRNA delivery with its unique azido-acetal linker, enabling rapid hydrolysis in endosomes (pH ~6.0). This breakthrough technology ensures efficient endosomal escape, significantly enhancing mRNA delivery to target cells. ADA-LNPs excel in delivering mRNA to the spleen and liver, making them ideal for immune-related therapies.By degrading into biocompatible byproducts, ADA minimizes long-term tissue persistence and toxicity.ADA-LNPs outperform traditional LNPs, delivering mRNA more effectively to immune cells like macrophages and B cells.
More description
|
.png)
|
| DC60636 | Acid-degradable Cationic Lipid (ADC) Featured |
Acid-degradable Cationic Lipid (ADC) composed of cationic lipid is synthesized with the azido-acetal linker and used to generate RD-LNPs, which significantly improves the performance of LNP-mRNA complexes in vitro and in vivo.
More description
|
.png)
|
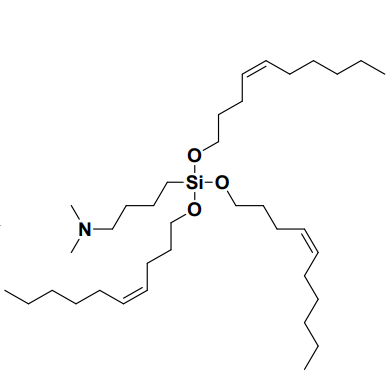
| DC60564 | Lipid GVS-18-B6 Featured |
GVS-18-B6 is a silicon ether-based ionizable lipid developed by Genevant, optimized for mRNA-LNP delivery, characterized by a short-chain trialkyl structure with three C10 alkyl chains (including a cis double bond) and a dimethylamino (DMA) head group linked via a 4-carbon spacer (pKa ~6.15). Its LNP formulations exhibit a narrow particle size distribution (89 nm, PDI=0.06), high mRNA encapsulation efficiency (90%), and pH-dependent surface charge (−0.11 mV at pH 7.5 vs. +2.69 mV at pH 5.5), facilitating endosomal escape. In vivo, GVS-18-B6 demonstrated superior liver-specific mRNA expression (5.30×10⁷ pg EGFP/g liver, 2.6× higher than MC3) with minimal spleen accumulation (liver/spleen ratio 92:1 vs. MC3’s 10:1), attributed to rapid non-enzymatic hydrolysis of its silicon ether bonds. This mechanism enables near-complete hepatic clearance within 6 hours in mice and 24 hours in NHPs, avoiding long-term organ retention (MC3 retained 25% in liver after 28 days). Compared to benchmarks (MC3, SM-102, LP-01), GVS-18-B6 showed enhanced potency in RBC hemolysis assays (pH 6.2), indicating earlier endosomal membrane disruption, and maintained stability through 12-month frozen storage or repeated freeze-thaw cycles. Toxicity profiling revealed minimal immunogenicity (MCP-1 levels 0.58×10⁶ vs. MC3’s 1.10×10⁶) and no ALT/AST elevation or anti-PEG antibody induction in NHPs after repeated dosing. Its species-agnostic clearance, low off-target effects, and high tolerability (6 mg/kg dose in mice) position GVS-18-B6 as a leading candidate for chronic disease therapies requiring frequent mRNA administration.
More description
|
|
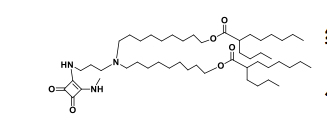
| DC67219 | Lipid 29 analogue-3 Featured |
Lipid 29 analogue-3 is an ionizable lipid designed for the delivery of RNA-based therapeutics, such as mRNA or siRNA.
More description
|
|
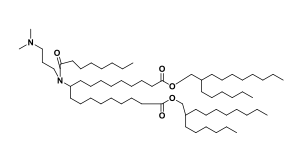
| DC67214 | Acuitas II-12 Featured |
Acuitas II-12 is an novel ionizable amine lipid used for mRNA delivery from Acuitas Therapeutics patent WO2016176330A1
More description
|
|
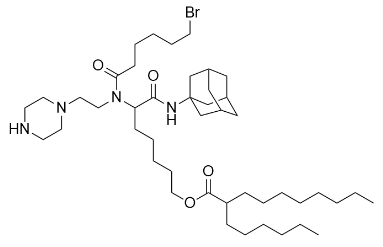
| DC60793 | LUMI6 Featured |
The LUMI-6 lipid, autonomously designed via the LUMI-lab platform, is a brominated ionizable lipid optimized for mRNA delivery. Formulated at a molar ratio of 35:28:34.5:2 (LUMI-6:DOTAP:cholesterol:C14-PEG2000), LNPs exhibit uniform physicochemical properties, including a hydrodynamic diameter of ~80 nm, polydispersity index (PDI) <0.2, and robust mRNA encapsulation efficiency. In vitro, LUMI-6 LNPs demonstrated 1.8-fold higher transfection potency in human bronchial epithelial cells compared to its debrominated counterpart (LUMI-6D), with minimal cytotoxicity confirmed by CCK-8 assays. In vivo, pulmonary delivery of CRISPR-Cas9 mRNA via LUMI-6 LNPs achieved 20.3% gene editing efficiency in murine lung epithelial cells, surpassing SM-102 (Moderna’s clinical benchmark) and demonstrating preferential tropism for lung epithelium over endothelial cells—critical for inhaled therapies targeting cystic fibrosis and surfactant disorders. The brominated tail enhances endosomal escape through optimized protonation dynamics, though explicit pKa values remain unmeasured. Synthesized via high-throughput combinatorial chemistry and refined through AI-driven active learning, LUMI-6 combines scalable production with organ-selective delivery, positioning it as a transformative platform for pulmonary nucleic acid therapeutics.
More description
|
|
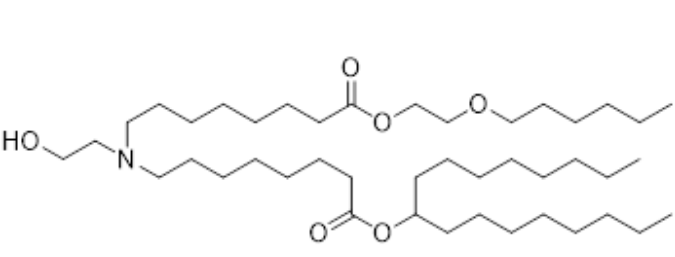
| DC153158 | ND-O1 (SM-86 Analog-2) Featured |
ND-O1 (SM-86 Analog-2) is a novel ionizable lipid designed to improve the delivery of siRNA via lipid nanoparticles (LNPs) for treating liver fibrosis. It is derived from SM-86 (structurally similar to SM-102, used in COVID-19 mRNA vaccines) but incorporates an ether bond within its hydrophobic tail, a first-of-its-kind modification aimed at enhancing delivery efficiency. In Vitro Efficiency: ND-O1 LNPs (LNP-O1) showed significantly higher siRNA transfection efficiency in activated fibroblasts compared to Lipid 5 LNPs (LNP-M). In Vivo Efficacy: In a CCl4-induced liver fibrosis mouse model, LNP-O1/siHSP47 (loaded with HSP47-targeting siRNA) reduced HSP47 expression by ~84%, threefold more effective than LNP-M. This led to a dramatic reduction in collagen deposition and marked improvement in liver fibrosis. Safety: The ether bond modification did not introduce additional toxicity, maintaining biocompatibility. ND-O1 represents a breakthrough in ionizable lipid design, demonstrating that strategic placement of ether bonds in hydrophobic tails can enhance LNP performance without compromising safety. Its success highlights its potential for clinical translation in RNA-based therapies for liver fibrosis and other hepatic diseases.
More description
|
|
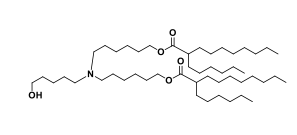
| DC67215 | Acuitas Lipid III-7 Featured |
Acuitas Lipid III-7 is an novel ionizable amine lipid used for mRNA delivery from Acuitas Therapeutics patent US 10,166,298 B2.
More description
|
|
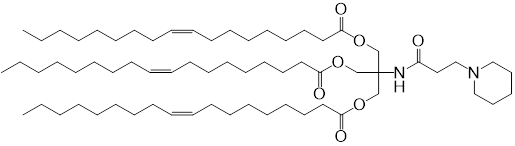
| DC60821 | Lipid TOT-5 Featured |
TOT-5, a tri-oleoyl-Tris ionizable lipid (pKa 6.2), enables splenic B cell-targeted mRNA delivery via 15% DSPC-incorporated LNPs. Its charge-neutral, hydrophobic surface minimizes hepatic ApoE uptake and enhances complement C3 adsorption, facilitating CD21/35-mediated uptake by marginal zone B cells. In vivo, intravenous 15%DSPC-LNPs showed 8-fold higher spleen-to-liver luciferase expression vs 3%DSPC, with anti-CD21/35 blocking 60% B cell uptake. Intramuscular administration induced robust OVA-specific IgG (10^5 titer) and CTL responses (3.5% tetramer+ CD8+ T cells) while reducing hepatotoxicity (ALT/AST levels ≤40 U/L vs SM-102-LNPs' 80-120 U/L). Cryo-ET confirmed stable lamellar structures (80-100 nm, ζ-potential -2 mV). This formulation achieves safe, ligand-free splenic targeting for mRNA vaccines.
More description
|
|
| DC60605 | Lipid 119-23 Featured |
Lipid 119-23 is an ionizable lipid for mRNA delivery. 119-23 LNP exhibits an enhanced capability to express functional mCre in several categories of immune cells, spanning the liver, spleen and lung.
More description
|
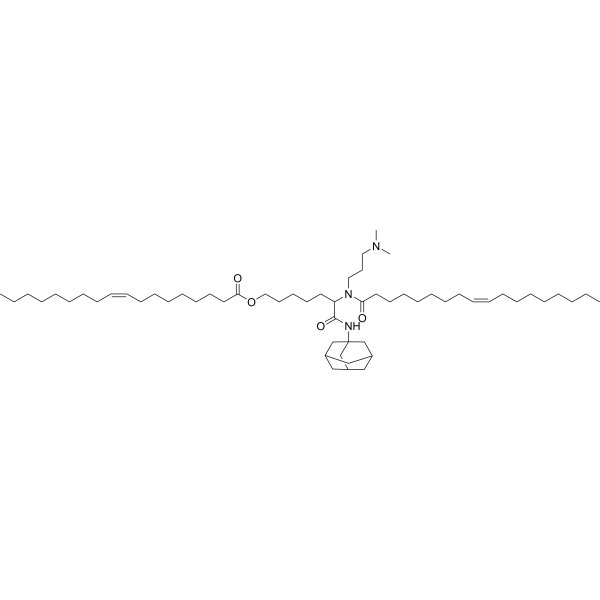
|
| DC82102 | PPZ-A10 Featured |
PPZ-A10 is an ionizable cationic lipid.It has been used in the generation of lipid nanoparticles (LNPs) for the delivery of siRNA and mRNA in vitro and in vivo. Intraperitoneal administration of LNPs containing PPZ-A10 and encapsulating an mRNA reporter preferentially accumulates in hepatic Kupffer cells and splenic macrophages in mice.
More description
|
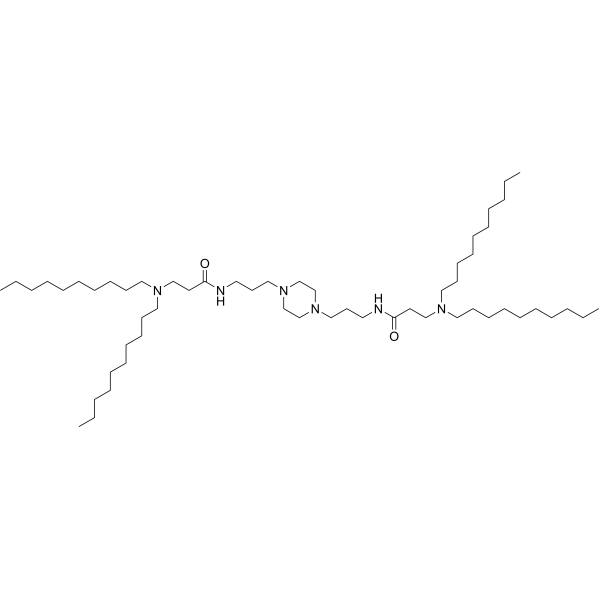
|
| DC82105 | 93-O17O Featured |
93-O17O is a chalcogen-containing ionizable cationic lipidoid. It has been used in the generation of lipid nanoparticles (LNPs). LNPs containing 93-O17O localize to the spleen after intravenous injection into mice.LNPs containing 93-O17O have been used for the delivery of Cre recombinase and ribonucleoproteins for genome editing in mice and for the intratumoral delivery of cGAMP to enhance cross-presentation of tumor antigens.
More description
|

|
| DC66219 | Lipid 88 Featured |
Lipid88 is a high-performance, novel ionizable lipid component engineered for advanced mRNA-LNP vaccine delivery. LNP88 formulation demonstrates superior biodistribution, achieving >10-fold higher transfection efficiency in spleen and lymph nodes compared to benchmark lipids like ALC-0315 via intramuscular delivery. When encapsulating antigen-encoding mRNA (e.g., optimized mCSA construct), Lipid-88 based LNPs drive robust humoral and cellular immunity, enabling complete protection against challenging SARS-CoV-2 variants (WA1/2020, Omicron BA.1, BQ.1) in preclinical models. Its design prioritizes potent immunogenicity with favorable safety profiles.
More description
|

|
| DC59010 | C14-4 Featured |
C14-4 (C14-494,Lipid B-4,Lipid B4) is a novel ionizable lipid with the highest T-cell transfection efficiency and low cytotoxicity.The C14-4 ionizable lipid has been explored for CAR-T therapy.To screen the excellent formulations for mRNA delivery, a
lipid library of 24 ionizable lipids was constructed to make
iLNPs, which were used to deliver luciferase mRNA into
Jurkat cells.[115] The optimal iLNPs formulation was C14-4
iLNPs (C14-4 ionizable lipid, DOPE, chol, and PEG at a molar
ratio of 35%, 16%, 46.5%, and 2.5%) (Figure 6c). The optimal
dose of luciferase mRNA for C14-4 iLNPs was 30 ng.
Compared with electroporated CAR T cells, the CAR T cells engineered
via C14-4 iLNPs showed potent cancer-killing activity
when they were cocultured with Nalm-6 acute lymphoblastic leukemia
cells. To obtain a safer and more effective CAR mRNA
delivery vehicle, the orthogonal design provided 256 potential
formulations, and 16 representative iLNPs formulations were
evaluated.Through evaluating the safety, delivery efficiency,
and transfection efficiency of 16 iLNPs, the formulation B10
(C14-4 ionizable lipid, DOPE, chol, PEG at a molar ratio of
40%, 30%, 25%, and 2.5%) was screened out as the optimal performing formulation. The luciferase expression based on B10
formulation was increased threefold than the initial formulation.
Reducing the accumulation and clearance of iLNPs in the liver
can increase the expression of CAR mRNA in T cells, further
improving the therapeutic effect of CAR-T. Studies have shown
that cholesterol analogs can alter the mechanisms of intracellular
circulation and enhance the delivery of mRNA, which may be
related to the reduced recognition of iLNPs by the Niemann
Pick C1 (NPC1) enzyme.The addition of a hydroxyl
group to various locations in the cholesterol molecule can alter
the binding kinetics between the modified cholesterol and NPC1,
and reduced NPC1 recognition of cholesterol. The results
showed that replacement of 25% and 50% 7 α-hydroxycholesterol
for cholesterol in iLNPs improved mRNA delivery to
primary human T cells in vitro by 1.8-fold and twofold,
respectively.C14-4 is one of the ionizable lipids to efficiently deliver mRNA
to Jurkat cells or primary human T cells. It will effectively promote
the development of mRNA delivery by iLNPs for CAR-T
therapy.
More description
|

|
| DC60808 | 503O8,12 Featured |
503O8,12 is an ionizable lipidoid synthesized via Michael addition, combining a hydrophilic amine headgroup ("503" series) with two hydrophobic branched acrylate tails (C8 and C12 chains, likely with unsaturated bonds). Its design emphasizes organ-specific delivery, exhibiting spleen-tropic targeting in vivo.
More description
|
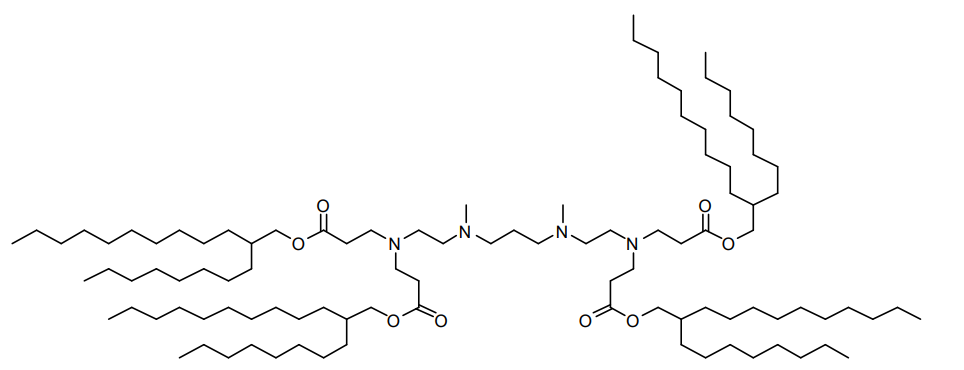
|
| DC67120 | YSK12-C4 (YSK12-MEND) Featured |
YSK 12C4 is an ionizable cationic lipid primarily used to enhance siRNA cellular delivery via multifunctional envelope-type nanodevices (MEND). YSK 12C4 promotes siRNA uptake and endosomal escape, effectively silencing genes in human immune cell lines.
More description
|
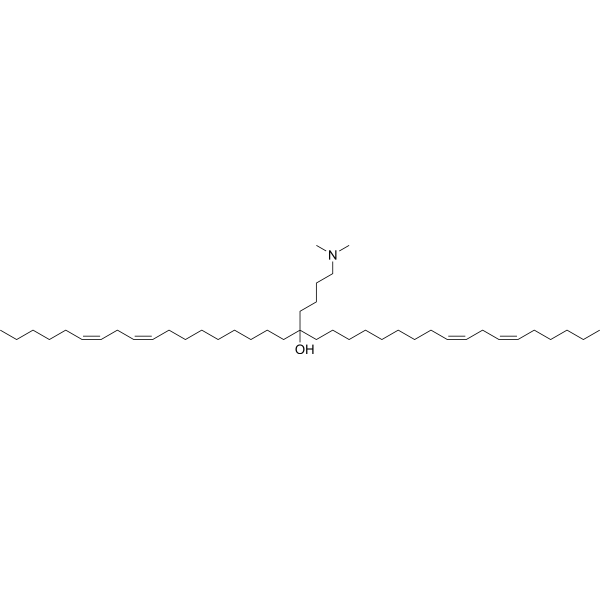
|
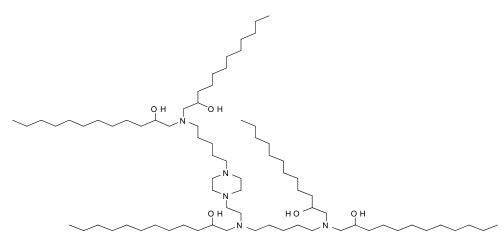
| DC60503 | C12-A1 Featured |
Lipid C12-A1 is an ionizable lipid. C12-A1-LPN is a potent and safe LNP platform to deliver Foxp3 mRNA to CD4+ T cells to engineer immunosuppressive FP3T cells. C12-A1 has a slightly lower average cell viability than C14-A1.
More description
|
|
| DC67521 | Lipid TD5 Featured |
TD5 is a brain-targeting lipid nanoparticle (BLNP) engineered for efficient mRNA delivery to the central nervous system (CNS) via intrathecal injection. It incorporates a tryptamine-derived ionizable lipid headgroup, myristic acid hydrocarbon tails, and a biodegradable carbonate ester linker, enabling pH-dependent mRNA encapsulation (81.7% efficiency) and brain cell-specific targeting. With a hydrodynamic diameter of 107.5 nm, near-neutral pKa (7.30), and mild positive charge, TD 5 demonstrates superior CNS tropism through serotonin receptor (5-HT1A)-mediated endocytosis. In vitro, TD-5 achieved 80.8% GFP expression in SH-SY5Y neuronal cells, outperforming MC3 LNPs by 50-fold. Following intrathecal administration in mice, TD-5 mediated GFP expression in 29.6% of neurons and 38.1% of astrocytes brain-wide, with 10-fold higher CNS specificity than peripheral organs. Genome editing studies showed TD5-delivered Cas9/sgRNA induced tdTomato activation in ≈30% of neurons and 40% of astrocytes across key brain regions. Safety profiling revealed minimal systemic immune responses (lower IL-6, IL-12p40 vs MC3 LNPs), normal hepatic/renal biomarkers, and no histopathological toxicity. The optimized structure balances myristic chain hydrophobicity for membrane interaction, ionizable amines for mRNA complexation, and tryptamine-mediated targeting for enhanced CNS uptake, establishing TD5 as a promising platform for CNS gene therapies.
More description
|

|
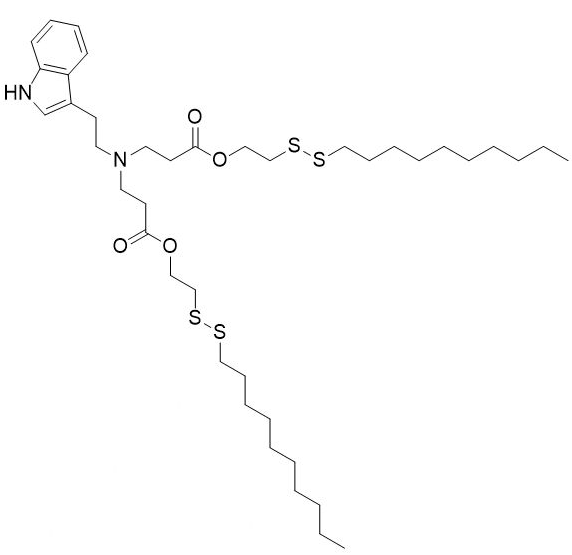
| DC60212 | NT1-O14B Featured |
NT1-O14B is a tryptamine-containing cationic lipidoid.1 It has been used in combination with other lipids in the formation of lipid nanoparticles (LNPs). Intravenous administration of LNPs containing NT1-O14B and encapsulating antisense nucleotides against tau decreases tau brain levels in mice.
More description
|
|
| DC41043 | NT1-O12B Featured |
NT1-O12B, an endogenous chemical and a neurotransmitter-derived lipidoid (NT-lipidoid), is an effective carrier for enhanced brain delivery of several blood-brain barrier (BBB)-impermeable cargos. Doping NT1-O12B into BBB-impermeable lipid nanoparticles (LNPs) gives the LNPs the ability to cross the BBB. NT-lipidoids formulation not only facilitate cargo crossing of the BBB, but also delivery of the cargo into neuronal cells for functional gene silencing or gene recombination.
More description
|

|
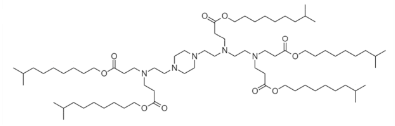
| DC60545 | 200Oi10 Featured |
200Oi10 is an ionizable lipidoid used in lipid nanoparticles (LNPs) for RNA delivery. Structurally, it features ester-conjugated cleavable lipid tails, enhancing biodegradability and reducing toxicity compared to non-cleavable analogs. Preclinical studies show that 200Oi10-based LNPs primarily accumulate in the liver (97.7%) after intravenous administration. However, intraperitoneal injection redirects biodistribution, achieving 46.4% pancreatic uptake, which can be further amplified by incorporating cationic lipids like DOTAP. This unique tropism enables pancreas-targeted mRNA delivery. 200Oi10's ester linkages promote rapid clearance, improving biocompatibility while maintaining siRNA/mRNA delivery efficiency. Its design exemplifies the use of degradable lipidoids to balance organ specificity, efficacy, and safety in RNA therapeutics.
More description
|
|
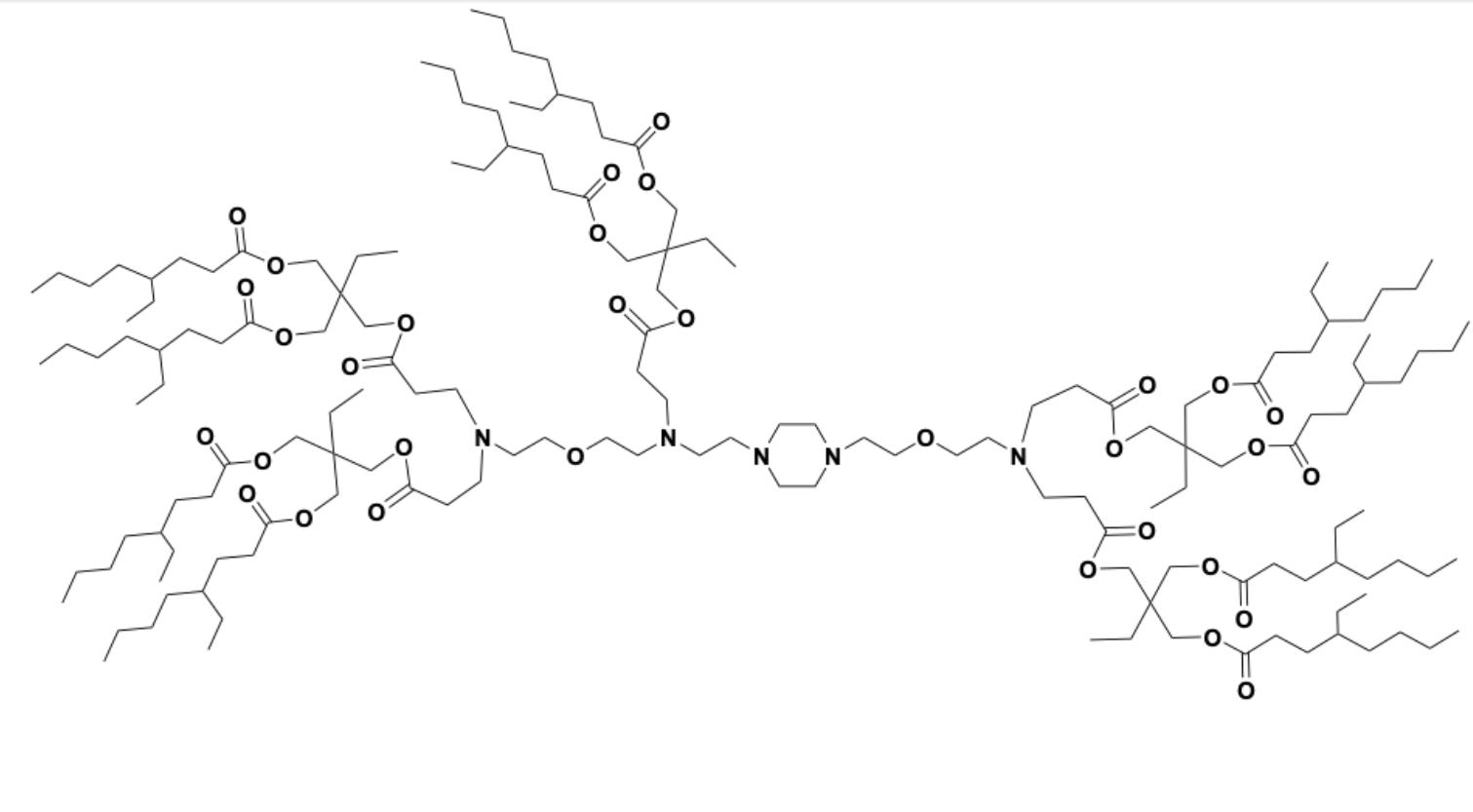
| DC60800 | 18-2-9b2 Featured |
18-2-9b2 is a dendron-like degradable ionizable lipid which facilitates mRNA delivery to splenic macrophages. 18-2-9b2 LNP encapsulating therapeutic BTB domain and CNC homologue 1 (BACH1) mRNA exhibited proficient BACH1 expression and subsequent Spic downregulation in splenic red pulp macrophages (RPM) in a Spic-GFP transgene model.
More description
|
|
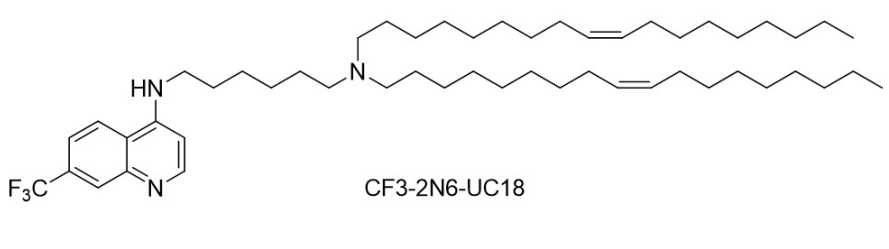
| DC60843 | CF3-2N6-UC18 Featured |
CF3-2N6-UC18 is a rationally designed chloroquine-inspired ionizable lipid that enables robust mRNA delivery and genome editing. It integrates three modular components: a 7-trifluoromethyl-substituted quinoline scaffold (mimicking chloroquine’s endosomolytic properties), a hexamethylenediamine linker with two ionizable nitrogen atoms (pH-responsive protonation), and two unsaturated oleyl (C18:1) hydrophobic tails (enhancing membrane fusion and nanoparticle stability). This lipid self-assembles into ecoLNPs (endosomolytic chloroquine-like lipid nanoparticles) with spherical morphology (~200 nm diameter, 98% mRNA encapsulation). Its pH-sensitive activity triggers endosomal escape through dual mechanisms: proton sponge effect (buffering endo-lysosomal pH) and saposin B-mediated membrane disruption (molecular docking confirms chloroquine-like binding to lysosomal saposin B). In vitro, ecoLNPs outperform commercial reagents (18.9-fold higher mRNA delivery than Lipofectamine 2000) and penetrate 3D cell models. They resist serum/RNase degradation and retain >90% activity after 7-day storage at 4°C. In vivo, ecoLNPs achieve tissue-specific mRNA expression via multiple routes (intravenous, intramuscular, etc.), with strong lymph node tropism (90.2% after intramuscular injection) comparable to SM-102 LNPs (Moderna’s COVID-19 vaccine carrier). They mediate efficient Cre mRNA-driven recombination and CRISPR-Cas9 editing in transgenic mice. CF3-2N6-UC18’s modular design, stability, and dual endosomal escape strategies position it as a versatile platform for mRNA vaccines, gene therapy, and genome editing applications.
More description
|
|