| Cas No.: | |
| Chemical Name: | Lipid PL40 |
| Synonyms: | Lipid-PL40, PL 40 ,PL-40 |
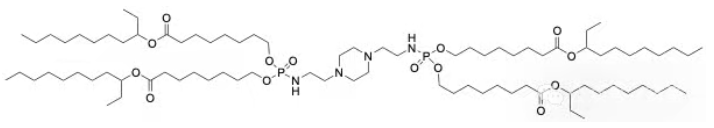
| SMILES: | CCCCCCCCC(CC)OC(=O)CCCCCCCOP(=O)(NCCN1CCN(CCNP(=O)(OCCCCCCCC(=O)OC(CC)CCCCCCCC)OCCCCCCCC(=O)OC(CC)CCCCCCCC)CC1)OCCCCCCCC(=O)OC(CC)CCCCCCCC |
| Formula: | C84H166O14N4P2 |
| M.Wt: | 1518.21 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | Cardiolipin-mimic lipid nanoparticles without antibody modification delivered senolytic in vivo CAR-T therapy for inflamm-aging-Cell Rep Med. 2025 Jul 15;6(7):102209. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.